Types of proteins and their functions in the human body. Protective function of proteins. Structure and functions of proteins Functions of proteins in a cell table
I. Table 2. Classification of proteins according to their structure.
| Protein class | Characteristic | Function |
| Fibrillar | The most important is the secondary structure (the tertiary structure is almost not expressed) Insoluble in water Characterized by high mechanical strength Long parallel polypeptide chains, cross-linked to each other, form long fibers or layered structures | Perform structural functions. This group includes, for example, collagen (tendons, bones, connective tissue), myosin (muscles), fibroin (silk, spider webs), keratin (hair, horns, nails, feathers). |
| Globular | The tertiary structure is most important Polypeptide chains are folded into compact globules Soluble | Perform the functions of enzymes, antibodies, and in some cases hormones (for example, insulin), as well as a number of other important functions |
| Intermediate | Fibrillar in nature, but soluble | An example is fibrinogen, which is converted into insoluble fibrin during blood clotting |
II. Classification of proteins according to their composition.
Simple Complex
Consist only of amino acids Consist of globular proteins and non-protein
material. The non-white part is called
prosthetic group.
Table 3. Complex proteins.
| Name | Prosthetic group | Example |
| Phosphoproteins | Phosphoric acid | Milk casein Egg yolk vitellin |
| Glycoproteins | Carbohydrate | Membrane Components Mucin (component of saliva) |
| Nucleoproteins | Nucleic acid | Components of viruses Chromosomes Ribosomes |
| Chromoproteins | Pigment | Hemoglobin - heme (iron-containing pigment) Phytochrome (pigment of respiratory origin) Cytochrome (respiratory pigment) |
| Lipoproteins | Lipid | Membrane components Blood lipoproteins are a transport form of lipids |
| Metalloproteins | Metal | Nitrareductase is an enzyme that catalyzes the conversion of sodium into nitrite in plants. |
III. Table 4. Classification of proteins by function.
| Protein class | Examples | Localization/function |
| Structural proteins | Collagen Keratin Elastin | Component of connective tissue, bones, tendons, cartilage Skin, feathers, nails, hair, horns Ligaments |
| Enzymes | Trypsin Ribulose biphosphate carboxylase | Catalyzes the hydrolysis of proteins Catalyzes (addition of CO 2) during photosynthesis |
| Hormones | Insulin Glucagon ACTH | Regulate glucose metabolism Stimulates the growth and activity of the adrenal cortex |
| Respiratory pigments | Hemoglobin Myoglobin | Carries O 2 in the blood of vertebrates Serves for storing O 2 in muscles |
| Transport proteins | Albumen | Serves for transport of fatty acids and lipids in the blood |
| Protective proteins | Antibodies Fibrinogen Thrombin | Form complexes with foreign proteins Precursor of fibrin during blood clotting Participates in the process of blood clotting |
| Contractile proteins | myosin Actin | Movable muscle filaments Fixed muscle filaments |
| Spare proteins | Egg Albumin Casein | Egg white Milk white |
| Toxins | snake poison | Enzymes |
Enzymes(enzymes) are specific proteins that are present in all living organisms and play the role of biological catalysts.
Enzymes speed up reactions without changing the overall outcome.
Enzymes are highly specific: each enzyme catalyzes a specific type of chemical reaction in cells. This ensures fine regulation of all vital processes (respiration, digestion, photosynthesis, etc.)
Example: the enzyme urease catalyzes the breakdown of only urea, without exerting catalytic pressure on structurally related compounds.
Enzyme activity is limited to a fairly narrow temperature range (35-45°C), beyond which the activity decreases and disappears. Enzymes are active at physiological Ph values, i.e. in a slightly alkaline environment.
According to their spatial organization, enzymes consist of several domains and usually have a quaternary structure.
Enzymes may also contain non-protein components. The protein part is called apoenzyme , and non-protein – cofactor (if it is a simple inorganic substance, for example Zn 2+, Mg 2+) or coenzyme (coenzyme) ) (if we are talking about organic compounds).
The precursors of many coenzymes are vitamins.
Example: pantathenic acid is a precursor to coenzyme A, which plays an important role in metabolism.
Enzyme molecules contain a so-called active center . It consists of two sections - sorption And catalytic . The first is responsible for the binding of enzymes to substrate molecules, and the second is responsible for the actual act of catalysis.
The name of the enzymes contains the name of the substrate on which the enzyme acts and the ending “-aza”.
ü cellulose - catalyzes the hydrolysis of cellulose to monosaccharides.
ü protease – hydrolyzes proteins to amino acids.
According to this principle, all enzymes are divided into 6 classes.
Oxidoreductases catalyze redox reactions, carrying out the transfer of H and O atoms and electrons from one substance to another, while oxidizing the first and reducing the second. This group of enzymes is involved in all biological oxidation processes.
Example: in breathing
AN + B ↔A + VN (oxidative)
A + O ↔ AO (reductive)
Transferases catalyze the transfer of a group of atoms (methyl, acyl, phosphate and amino groups) from one substance to another.
Example: under the pressure of phosphotransferases, phosphoric acid residues are transferred from ATP to glucose and fructose: ATP + glucose ↔ glucose – 6 – phosphate + ADP.
Hydrolases accelerate reactions, break down complex organic compounds into simpler ones by adding water molecules at the site where chemical bonds are broken. This kind of splitting is called hydrolysis .
These include amylase (hydrolyzes starch), lipase (breaks down fats), etc.:
AB + H 2 O↔AON + VN
Lyases catalyze non-hydrolytic additions to the substrate and the detachment of a group of atoms from it. In this case, there may be a break in the C – C, C – N, C – O, C – S bonds.
Example: removal of a carboxyl group by decarboxylase
CH 3 – C – C ↔ CO 2 + CH 3 – C
Isomerases carry out intramolecular rearrangements, i.e. catalyze the conversion of one isomer to another:
glucose – 6 – phosphosate ↔ glucose – 1 – phosphate
Lipases( synthetases) catalyze the reactions of joining two molecules with the formation of new bonds C – O, C – S, P – N, C – C, using the energy of ATP.
Lipases include a group of enzymes that catalyze the addition of amino acid residues to t-RNA. These synthetases play an important role in the process of protein synthesis.
Example: the enzyme valine - t-RNA - synthetase, under its action, a valine-t-RNA complex is formed:
ATP + valine + tRNA ↔ ADP + H 3 PO 4 + valine-tRNA
Basic biological functions of proteins
Squirrels are part of every cell and make up about 50% of its dry mass. They play a key role in metabolism and carry out the most important biological functions that form the basis of the life activity of all organisms.Among the wide variety of functions performed by proteins, structural, or plastic, and catalytic ones are of primary importance. These are universal functions, since they are inherent in all living organisms - from microbial cells to higher representatives of plants and animals, including humans.
Functions of proteins
Structural proteins form the framework of intracellular organelles and extracellular structures, and also participate in the stabilization of cell membranes. Thus, all cells, and therefore tissues, and the body as a whole are built from proteins, which determine the structure and shape of organelles, cells, tissues and the entire organism.Examples of structural proteins are collagen and elastin, which form the basis of connective and bone tissues of higher animals and humans. When collagen is boiled, gelatin is formed, which is used in the food industry. Structural proteins include keratins, which form the basis of the horny derivatives of the epidermis of the skin - hair, nails, wool, claws, horns, hooves, feathers, beaks, shells, needles, etc., as well as silk fibroin and spider webs.
Catalytically active proteins are enzymes. They accelerate almost all chemical reactions occurring in living organisms, thereby providing the speed necessary for metabolic processes.
Many proteins inherent in certain groups of living organisms perform specific functions, among which the most important are transport, regulatory, protective, receptor, contractile, reserve, etc.
Transport proteins transport various molecules and ions within the body. For example, hemoglobin transports oxygen from the lungs to tissues and carbon dioxide from tissues to the lungs in animals and humans, and myoglobin transports oxygen to mitochondria inside red muscle cells. Serum albumin is a carrier of many substances transported by blood - fatty acids, some metal ions, etc.
Proteins in this group also include specific proteins with the help of which various substances move through cell membranes.
Regulatory proteins participate in the regulation of metabolism both inside cells and in the whole organism. For example, such complex processes as the biosynthesis of proteins and nucleic acids occur under the strict “control” of many regulatory proteins. Specific protein inhibitors regulate the activity of many enzymes.
A characteristic feature of this group of proteins is the ability to influence the fundamental mechanisms of metabolism. For example, insulin is a protein hormone produced by the pancreas of humans and animals. It serves as a signaling substance that regulates the concentration of glucose in the blood. With a lack of insulin in the body, a serious disease develops - diabetes mellitus.
Protective proteins form the defense system of living organisms. For example, immunoglobulins (antibodies) and interferons protect the body from the penetration of various antigens into its internal environment (from the English antibody generator - antibody manufacturer) - viruses, bacteria, foreign cells and tissues. They are produced by animal organisms in response to an attack by pathogens or foreign particles, recognize and inactivate them, thereby protecting the body from the negative effects of antigens.
Protective function Fibrinogen, a protein of the blood coagulation system, also performs this function, preventing blood loss when blood vessels are damaged.
Receptor proteins perceive signals coming from the external environment and influence intracellular processes. For example, receptor proteins located on the surface of cell membranes selectively interact with regulatory molecules (for example, hormones); receptor proteins of the sense organs interact with signals such as light, color, taste, smell, sound, and transmit the resulting information to biological systems. Such proteins are rhodopsin, which is involved in the visual act, taste-sweet and olfactory proteins, etc.
Contractile proteins have mechanochemical properties, i.e. the ability to convert free chemical energy into mechanical work. For example, the muscle proteins myosin and actin mediate muscle contraction and relaxation.
Storage proteins are reserve material intended to nourish developing cells. Storage proteins are egg albumin, wheat gliadin, corn zein, milk casein, etc.
Spare proteins- the most important source of dietary protein for humans, especially the reserve proteins of plant seeds. These proteins have a significant biochemical difference from the proteins of the vegetative parts of plants (stems, leaves, roots). Being reserve proteins, seed proteins are inert in comparison with the proteins of stems, leaves and roots that actively function throughout the entire period of plant life. During seed germination, storage proteins are mobilized due to their hydrolysis.
Toxic proteins produced by some organisms as a defense against potential enemies. For example, they are found in the venoms of snakes, scorpions, plant seeds (ricin from castor beans, legume lectins, etc.), and in microorganisms (cholera, diphtheria toxins, etc.).
Continuation. See No. 11, 12, 13, 14/2005
Biology lessons in science classes
Advanced planning, grade 10
III. Consolidation of knowledge
Filling out the table “Levels of protein organization.”
Table 5. Levels of protein organization
Organization level |
Signs |
Connections involved in the formation of the structure |
Primary |
Linear sequence of amino acids in a polypeptide chain |
Covalent (peptide) bonds between the carboxyl group residue of one amino acid and the amino group residue of another amino acid |
Secondary |
Helix, -structure or helices with parameters other than -helices |
Hydrogen bonds between the residues of the carboxyl group of one amino acid and the residue of the amino group of another, four amino acid residues distant from the first; in the -structure, hydrogen bonds between the residues of carboxyl and amino groups of one chain and the residues of groups of the same name on the other chain; in spirals - similar to -spirals, but the distance between the turns is different |
Tertiary |
A globule formed as a result of compact folding of an α-helix; -structures laid in parallel layers; supercoil – several helices twisted together |
Ionic, disulfide bridges, hydrophobic, hydrogen |
Quaternary |
An aggregate of several globules. Characteristic only of proteins with a particularly complex structure |
Mainly intermolecular attractive forces, to a lesser extent - hydrogen, ionic and covalent forces |
IV. Homework
Study the textbook paragraph (proteins, their content in living matter, structure and properties of amino acids, formation of peptides, levels of protein organization, classification of proteins).
Lesson 10–11. Biological functions of proteins
Equipment: tables on general biology, diagrams and drawings illustrating the structure of proteins, protein classification scheme.
I. Test of knowledge
Working with cards
Card 1. A young biochemist, determining the nitrogen content in a pure protein preparation, received a value of 39.9%. How can you comment on this result?
Card 2. The protein hemoglobin is found in humans in two variants:
hemoglobin in the blood of a healthy person (... val-lay-ley-tre-pro-val-glu-liz...);
hemoglobin in the blood of a patient with sickle cell anemia (...val-lay-lay-tre-pro-glu-glu-liz...). What causes the disease?
Card 3. How to determine the number of possible amino acids in a protein based on molecular weight? What determines the possible error of this estimate?
Card 4. How many variants of polypeptide chains can there exist, including 20 amino acids and consisting of 50 amino acid residues? Out of 200 leftovers?
Card 5. Fill in the blanks in the text: “As a result of the interaction of various... and the formation of... bonds, a spiralized protein molecule forms... a structure, which, in turn, depends on... the structure of the protein, that is, on.. amino acids in a polypeptide molecule. The subunits of some proteins form... a structure. An example of such a protein is...”
Card 6. Ions of heavy metals (mercury, lead) and arsenic easily bind to sulfide groups of proteins. Knowing the properties of sulfides of these metals, explain what will happen to the protein when combined with these metals. Why are heavy metals poisons for the body?
1. Proteins, their content in living matter, molecular weight.
2. Proteins are non-periodic polymers. Structure and properties of amino acids. Peptide formation.
3. Primary and secondary structures of the protein molecule.
4. Tertiary and quaternary protein structures.
5. Classification of proteins.
II. Learning new material
1. Denaturation and other properties of proteinsProteins are extremely diverse in their physical and chemical properties. What is the reason for this? ( Conversation.) Let us give examples of the diversity of properties of proteins.
1. There are proteins that are soluble (for example, fibrinogen) and insoluble (for example, fibrin) in water.
2. There are proteins that are very stable (for example, keratin) and unstable (for example, the enzyme catalase with an easily changing structure).
3. Proteins have a variety of molecular forms - from filaments (myosin - muscle fiber protein) to balls (hemoglobin), etc.
But the structure and properties of a protein always correspond to the function it performs.
The most important property of all living systems, irritability, is based on the ability of proteins to reversibly change their structure in response to the action of physical and chemical factors. Since the secondary, tertiary and quaternary structures of a protein are created by generally weaker bonds than the primary, they are less stable. For example, when heated, they are easily destroyed. Moreover, although the protein retains its primary structure intact, it cannot perform its biological functions and becomes inactive. The process of destruction of the natural conformation of a protein, accompanied by loss of activity, is called denaturation. Breaking of some weak bonds, changes in conformation and properties also occur under the influence of physiological factors (for example, under the influence of hormones). In this way, the properties of proteins - enzymes, receptors, transporters - are regulated.
These structural changes are usually easily reversible. The reverse process of denaturation is called renaturation. This property of proteins is widely used in the medical and food industries for the preparation of certain medical products, such as antibiotics, vaccines, serums, enzymes; to obtain food concentrates that retain their nutritional properties for a long time in dried form.
If restoration of the spatial configuration of the protein is impossible, then denaturation is considered irreversible. This usually occurs when a large number of bonds are broken, for example when boiling eggs.
Thus, proteins have a complex structure, various shapes and composition. This makes their properties diverse. This, in turn, allows proteins to perform numerous biological functions.
2. Biological functions of proteins
Proteins perform a number of important functions in the cell and body, the main ones of which are the following.
1. Structural (construction). Proteins are part of all cell membranes and cell organelles, as well as extracellular structures. An example of a protein that performs a structural function is keratin. This protein consists of hair, wool, horns, hooves, and the upper dead layer of skin. In the deeper layers of the skin there are pads of proteins collagen And elastin. It is these proteins that provide the strength and elasticity of the skin. They are also contained in ligaments that connect muscles to joints and joints to each other.
2. Enzymatic. Proteins are biological catalysts. For example, pepsin, trypsin, etc. (we will look at the properties of enzyme proteins in detail in the following lessons).
3. Motor. Special contractile proteins are involved in all types of cell and body movement: the formation of pseudopodia, the flickering of cilia and the beating of flagella in protozoa, muscle contraction in multicellular animals, leaf movement in plants, etc. Thus, muscle contraction is provided by muscle proteins actin And myosin, they also make it possible for the amoeba to crawl.
4. Transport. There are various transport proteins in the blood, in the outer cell membranes, in the cytoplasm and nuclei of cells. There are transporter proteins in the blood that recognize and bind certain hormones and carry them to target cells. The outer cell membranes contain transporter proteins that provide active and strictly selective transport of sugars, amino acids, and various ions into and out of the cell. Other transport proteins are also known, for example hemoglobin And hemocyanin, carrying oxygen, and myoglobin, which retains oxygen in the muscles.
5. Protective. In response to the penetration of foreign proteins or microorganisms with antigenic properties into the body, blood lymphocytes form special proteins - antibodies that can bind and neutralize them. Saliva and tears contain protein lysozyme– an enzyme that destroys bacterial cell walls. If a microbe gets on the mucous membrane of the eyes or oral cavity, its shell is destroyed by the action of lysozyme, and then protective cells can easily cope with it. Fibrin And thrombin help stop bleeding.
6. Energy (nutritional). Proteins can be broken down, oxidized and provide the energy needed for life. True, this is not very profitable: the energy value of proteins compared to fats is low and amounts to 17.6 kJ (4.1 kcal) energy per 1 g of protein. Typically, proteins are used for energy needs in extreme cases, when reserves of fats and carbohydrates are exhausted.
7. Regulatory. Many (though not all) hormones are proteins - for example, all the hormones of the pituitary gland, hypothalamus, pancreas ( insulin, glucagon) etc. Hormones act on the cell by binding to specific receptors. Each receptor recognizes only one hormone. The receptors for all hormones are proteins. Another example is proteins that regulate the formation and growth of individual organs and tissues during the development of the organism from the zygote. Phytochrome plants is a complex light-sensitive protein that regulates the photoperiodic response in plants.
8. Signal (receptor). The surface membrane of the cell contains embedded protein molecules that can change their tertiary structure in response to environmental factors. This is how signals are received from the external environment and commands are transmitted to the cell.
9. Storage. Thanks to proteins, certain substances can be stored in the body. Egg albumin serves as a water-storing protein in egg whites, milk casein is a source of energy, and protein ferritin retains iron in egg yolk, spleen and liver.
10. Toxic. Some proteins are toxins: cobra venom contains neurotoxin.
III. Consolidation of knowledge
Summarizing conversation while learning new material.
IV. Homework
Study the textbook paragraph (properties of proteins and their biological functions).
Lesson 12–13. Enzymes, their chemical composition and structure. Biological role of enzymes
Equipment: tables on general biology, diagrams and drawings illustrating the structure and mechanism of action of enzymes, a classification scheme for enzymes, equipment for laboratory work.
I. Test of knowledge
Working with cards
Card 1. It has been established that with sufficient caloric content of food, but in the absence of protein in it, pathological phenomena are observed in animals: growth stops, blood composition changes, etc. What is this connected with?
Card 2. Why are proteins called “carriers and organizers of life”?
Card 3. What structural features of a protein molecule enable it to perform many functions, for example transport, protective, energy?
Card 4. Fill in the blanks in the text: “Protective proteins are called... . They bind to..., entering the body and called.... Among thousands of different proteins... they recognize only one... and react with it. This mechanism of resistance to pathogens is called...”
Card 5. What similar functions do proteins, carbohydrates and lipids perform in living organisms?
Oral knowledge test on questions
1. Denaturation and other properties of proteins. Relationship between the structure, properties and functions of proteins.
2. Biological functions of proteins ( three students).
II. Learning new material
1. Enzymes and their importance in life processes
From your chemistry course you know what a catalyst is. This is a substance that speeds up a reaction, remaining unchanged at the end of the reaction (without being consumed). Biological catalysts are called enzymes(from lat. fermentum– fermentation, sourdough), or enzymes.
Almost all enzymes are proteins (but not all proteins are enzymes!). In recent years, it has become known that some RNA molecules also have the properties of enzymes.
The highly purified crystalline enzyme was first isolated in 1926 by the American biochemist J. Sumner. This enzyme was urease, which catalyzes the breakdown of urea. To date, more than 2 thousand enzymes are known, and their number continues to grow. Many of them are isolated from living cells and obtained in their pure form.
Thousands of reactions are constantly going on in the cell. If you mix organic and inorganic substances in a test tube in exactly the same proportions as in a living cell, but without enzymes, then almost no reactions will occur at a noticeable speed. It is thanks to enzymes that genetic information is realized and all metabolism is carried out.
The name of most enzymes is characterized by the suffix -ase, which is most often added to the name of the substrate - the substance with which the enzyme interacts.
2. Structure of enzymes
Compared to the molecular weight of the substrate, enzymes have a much larger mass. This discrepancy suggests that not the entire enzyme molecule is involved in catalysis. To understand this issue, you need to get acquainted with the structure of enzymes.
By structure, enzymes can be simple or complex proteins. In the second case, the enzyme contains, in addition to the protein part ( apoenzyme) there is an additional group of non-protein nature - an activator ( cofactor, or coenzyme), resulting in the formation of active holoenzyme. Enzyme activators are:
1) inorganic ions (for example, to activate the amylase enzyme found in saliva, chloride ions (Cl–) are required);
2) prosthetic groups (FAD, biotin) tightly bound to the substrate;
3) coenzymes (NAD, NADP, coenzyme A), loosely associated with the substrate.
The protein part and the non-protein component individually lack enzymatic activity, but when combined together they acquire the characteristic properties of an enzyme.
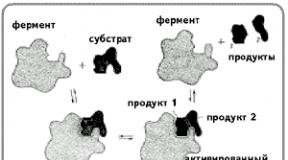
The protein part of enzymes contains active centers that are unique in their structure, which are a combination of certain amino acid residues strictly oriented in relation to each other (the structure of the active centers of a number of enzymes has now been deciphered). The active center interacts with the substrate molecule to form an “enzyme-substrate complex.” The "enzyme-substrate complex" then breaks down into the enzyme and the product or products of the reaction.
According to the hypothesis put forward in 1890 by E. Fisher, the substrate approaches the enzyme as key to the lock, i.e. the spatial configurations of the active site of the enzyme and the substrate correspond exactly ( complementary) each other. The substrate is compared to a “key” that fits the “lock” - the enzyme. Thus, the active center of lysozyme (the enzyme of saliva) has the appearance of a slit and in shape exactly corresponds to a fragment of a complex carbohydrate molecule of a bacterial bacillus, which is broken down under the action of this enzyme.
In 1959, D. Koshland put forward a hypothesis according to which the spatial correspondence of the structure of the substrate and the active center of the enzyme is created only at the moment of their interaction with each other. This hypothesis was called "hands and gloves" hypothesis(induced interaction hypothesis). This process of “dynamic recognition” is the most widely accepted hypothesis today.
3. Differences between enzymes and non-biological catalysts
Enzymes differ from non-biological catalysts in many ways.
1. Enzymes are much more efficient (10 4 –10 9 times). Thus, a single molecule of the catalase enzyme can break down 10 thousand molecules of hydrogen peroxide, which is toxic to cells, in one second:
2H 2 O 2 ––> 2H 2 O + O 2,
which occurs during the oxidation of various compounds in the body. Or another example confirming the high efficiency of enzymes: at room temperature, one urease molecule is capable of breaking down up to 30 thousand urea molecules in one second:
H 2 N–CO–NH 2 + H 2 O ––> CO 2 + 2NH 3.
Without a catalyst, this would have taken about 3 million years.
2. High specificity of enzyme action. Most enzymes act on only one or a very small number of “their” natural compounds (substrates). The specificity of enzymes is reflected by the formula "one enzyme - one substrate". Due to this, in living organisms many reactions are catalyzed independently.
3. Enzymes are subject to fine and precise regulation. The activity of an enzyme can increase or decrease with slight changes in the conditions in which it “works.”
4. Non-biological catalysts in most cases only work well at high temperatures. Enzymes, being present in cells in small quantities, work at normal temperature and pressure (although the scope of action of enzymes is limited, since high temperature causes denaturation). Since most enzymes are proteins, their activity is highest under physiologically normal conditions: t = 35–45 °C; slightly alkaline environment (although each enzyme has its own optimal pH value).
5. Enzymes form complexes - so-called biological conveyors. The process of breakdown or synthesis of any substance in a cell is usually divided into a number of chemical operations. Each operation is performed by a separate enzyme. A group of such enzymes constitutes a kind of biochemical conveyor belt.
6. Enzymes are capable of being regulated, i.e. “turn on” and “turn off” (however, this does not apply to all enzymes; for example, salivary amylase and a number of other digestive enzymes are not regulated). In most apoenzyme molecules there are sections that also recognize the final product that “comes off” the multienzyme conveyor. If there is too much of such a product, then the activity of the initial enzyme itself is inhibited by it, and vice versa, if there is not enough product, then the enzyme is activated. This is how many biochemical processes are regulated.
Thus, enzymes have a number of advantages over non-biological catalysts.
4. Mechanism of action of enzymes
Enzymes operate in living organisms according to the same laws as any catalysts. Enzyme catalysis is based on reducing the energy barrier (the so-called activation energy) due to the formation of intermediate complexes of the enzyme with the substrate. In the absence, for example, of amylase, the reaction between starch and water does not occur because the molecules do not have sufficient energy for this purpose. The enzyme speeds up the chemical process because in its presence, less energy is required to “start” a given reaction. Let us consider the mechanism of action of enzymes in more detail.
1. By catalyzing a reaction, the enzyme brings the molecules of “its” substrates closely together, so that those parts of the molecules that are to react are nearby.
2. The substrate, having joined the enzyme, changes somewhat. The enzyme can attract electrons, causing tension to occur in some of the bonds of the substrate molecule. This in turn increases the reactivity of the molecule, since the bonds between atoms are weakened and they are released more easily (this is supposed to be how the enzyme speeds up the reaction).
3. The enzyme “rips off” an atom (or atoms) from each of the substrates, after which the substrates are combined.
4. The separated atoms combine with each other and leave the enzyme. Now the enzyme is able to attach new molecules of substrates.
Most often, enzymes are confined to specific cellular structures. They retain their properties outside the body. Enzymes are successfully used in the baking, brewing, winemaking, leather, and chemical industries.
5. Classification of enzymes
Students work with the text of the textbook and fill out the table “The most important groups of enzymes” with subsequent checking.
Table 6. The most important groups of enzymes
Number and name of classes |
Catalyzed reactions |
|
1. Oxidoreductases 2. Transferases 3. Hydrolases 4. Lyases 5. Isomerases 6. Ligases (synthetases) |
Redox reactions: transfer of hydrogen or oxygen atoms or electrons from one substance to another Transfer of functional groups from one substance to another Hydrolysis: reactions that break down complex organic substances into simpler ones by adding water Non-hydrolytic addition or removal of functional groups Isomerization, i.e. conversion of isomers into each other Synthesis reactions using ATP energy |
Catalase decomposes hydrogen peroxide into water and molecular oxygen; cytochromes transfer and attach electrons to oxygen atoms during respiration and to protons during the reactions of the light phase of photosynthesis Under the action of phosphotransferases, phosphoric acid residues are transferred from ATP to glucose or fructose Amylase hydrolyzes starch to maltose; trypsin hydrolyzes proteins and peptides to amino acids Elimination of carboxyl groups by decarboxylases Interconversions of glucose and fructose in plants under the action of glucose phosphate isomerase Carboxylases catalyze the addition of carbon dioxide to organic acids |
III. Consolidation of knowledge
Laboratory work No. 1. “Study of the catalytic activity of the enzyme catalase in living tissues”Equipment: tripods, test tubes, bottles with a fresh 3% solution of hydrogen peroxide, plant and animal tissues, jars with water and elodea, microscopes, slides and cover glasses, tweezers and pipettes.
Progress
1. Pour 2 ml of hydrogen peroxide into test tubes containing raw meat, cooked meat, raw and boiled potatoes. Explain the phenomena you observe during the action of peroxide on living and dead tissues.
2. Place an Elodea leaf on a glass slide in a drop of water and examine under a microscope at low magnification the place where the leaf is torn from the stem.
3. Apply two drops of hydrogen peroxide to an elodea leaf, cover with a cover slip and examine under a microscope where the leaf is torn from the stem. Explain the rapid release of gas bubbles from damaged cells of the Elodea leaf.
4. Conclusions.
How does enzyme activity manifest itself in living and dead tissues? Why?
Does enzyme activity differ in living tissues of plants and animals?
How would you propose to measure the rate of decomposition of hydrogen peroxide?
Do you think all living organisms contain the enzyme catalase, which ensures the decomposition of hydrogen peroxide? Justify your answer.
IV. Homework
Study the textbook paragraph (enzymes, their meaning, structure, mechanism of action and classification).
Lesson 14–15. Nucleic acids are non-periodic polymers. Nucleotide structure. Formation of polynucleotides. Formation of a double-stranded DNA molecule. Principle of complementarity
Equipment: tables on general biology, diagrams and drawings illustrating the structure and mechanism of action of enzymes, a classification scheme for enzymes, a diagram of the structure of a nucleotide, a model of the structure of DNA.



I. Test of knowledge
Working with cards
Card 1. It is known that the rate of chemical reactions decreases by only 2–3 times when the temperature decreases by 10 °C. For greater stability of analyzed samples, biochemists store them at a low temperature. However, if a freezing person’s body temperature drops by at least 10 °C, this leads to serious, often irreversible consequences. Is there a contradiction here?
Card 2. From the notebooks of Kifa Mokievich: “Protease is an enzyme that breaks down peptide bonds in proteins. Amylase is an enzyme that breaks down glycosidic bonds in carbohydrates. It is known that all enzymes have extremely high specificity and fit the substrate like a key to a lock. Since the substrates of the enzymes are the same, then the enzymes themselves are the same. It follows that biochemists only need to study one amylase (say, from human saliva) and one protease (say, from washing powder) - after all, they are identical!” How could you object to Kifa Mokievich?
Card 3. A certain enzyme was isolated from rat tissue. Its solution at +4 °C retains catalytic activity for several weeks. After it was placed in a thermostat at +40 °C for 2 hours, it lost 50% of its activity. Is it true that after another 2 hours it would have become completely inactive? But in the body of a rat it is by no means +4 °C, but just +40 °C. So does she need such an unstable enzyme?
Card 4. Try to make a list of enzymes necessary for the existence of any cell. If you do not know the name of an enzyme, it is enough to indicate the reaction it catalyzes.
Card 5. An experimenter, studying the rate of protein breakdown by protease, discovered that over time it first increased several times, and then fell - until the enzyme activity was completely lost. How can this pattern be explained? Which proteases do you think have this property?
Card 6. Why might enzyme activity depend on pH?
Card 7. In what ways can a cell control the speed of chemical processes occurring in it? In what ways can the human body regulate the speed of chemical processes?
Card 8. How do you understand “catalytic (enzymatic) conveyor belt in a cell”? What is the advantage of a conveyor arrangement of enzyme molecules on a membrane compared to their free, random position in the cytoplasm?
Oral knowledge test on questions
1. Enzymes and their importance in life processes.
2. The structure of enzymes and the reason for their high specificity.
3. Differences between enzymes and non-biological catalysts.
4. Mechanism of action of enzymes.
5. Classification of enzymes.
II. Learning new material
1. Nucleic acids, their content in the cell, molecular sizes and molecular weight
Nucleic acids are natural high-molecular organic compounds, polynucleotides, which ensure the storage and transmission of hereditary (genetic) information in living organisms.
These organic compounds were discovered in 1869 by the Swiss physician I.F. Miescher in cells rich in nuclear material (leukocytes, salmon sperm). Nucleic acids are an integral part of cell nuclei, which is why they got their name (from the Latin. nucleus- core). In addition to the nucleus, nucleic acids are also found in the cytoplasm, centrioles, mitochondria, and chloroplasts.
There are two types of nucleic acids in nature: deoxyribonucleic acids (DNA) and ribonucleic acids (RNA). They differ in composition, structure and functions. DNA is a double-stranded molecule, while RNA is single-stranded. The content of nucleic acids in living matter is from 1 to 2%.
Nucleic acids are biopolymers that reach enormous sizes. The length of their molecules is hundreds of thousands of nanometers (1 nm = 10–9 m), which is thousands of times longer than the length of protein molecules. The DNA molecule is especially large. The molecular weight of nucleic acids reaches tens of millions and billions (10 5 –10 9). For example, the DNA mass of Escherichia coli is 2.5x109, and in the nucleus of a human germ cell (haploid set of chromosomes) the length of DNA molecules is 102 cm.
2. NC – non-periodic polymers. Types of nucleotides and their structure
Nucleic acids are non-periodic biopolymers, the polymer chains of which are formed by monomers called nucleotides. DNA and RNA molecules contain four types of nucleotides. DNA nucleotides are called deoxyribonucleotides, and RNA - ribonucleotides. The nucleotide composition of DNA and RNA is reflected in the table.
Table 7. Composition of DNA and RNA nucleotides
Let's look at the structure of a nucleotide. Nucleotides are complex organic compounds that include three components. The diagram of the structure of a DNA nucleotide is shown in the figure.

1. Nitrogenous bases have a cyclic structure, which, along with carbon atoms, includes atoms of other elements, in particular nitrogen. Due to the presence of nitrogen atoms in these compounds, they are called “nitrogenous”, and since they have alkaline properties, they are called “bases”. The nitrogenous bases of nucleic acids belong to the classes of pyrimidines and purines. Pyrimidine bases are derivatives of pyrimidine, which has one ring in its molecule. Pyrimidine bases are found in deoxyribonucleotides thymine And cytosine, and in the composition of ribonucleotides - cytosine And uracil. Uracil differs from thymine in the absence of a methyl group (–CH 3).
Purine bases are derivatives of purine, which has two rings. Purine bases include adenine And guanine. They are part of the nucleotides of both DNA and RNA.
2. Carbohydrate – pentose (C 5 ). This component also takes part in the formation of nucleotides. DNA nucleotides contain pentose - deoxyribose, and RNA nucleotides - ribose. The carbohydrate composition of nucleotides is reflected, as we see, in the names of nucleic acids: deoxyribonucleic and ribonucleic. Pentose compounds with a nitrogenous base are called “nucleosides.”
3. Phosphoric acid residue. Phosphate imparts acidic properties to nucleic acids.
So, a nucleotide consists of a nitrogenous base, pentose and phosphate. In the composition of nucleotides, on the one hand, a nitrogenous base is attached to the carbohydrate, and on the other, a phosphoric acid residue.
To be continued
These are polymers whose monomers are amino acids. They are mainly composed of carbon, hydrogen, oxygen and nitrogen.
In the majority of the studied proteins of all living organisms, 20 amino acids were identified that were involved in their construction.
During the synthesis of a protein molecule, different amino acids are added sequentially to each other, forming a chain, or polypeptide (subsequently it can be folded into a spiral or globule). The diversity of proteins is determined by which amino acids, in what quantity and in what order are included in the polypeptide chain. Two molecules that are identical in the number and composition of amino acids, but differ in the order of their arrangement, represent two different proteins. Not only species, but also individuals of the same species differ in a number of proteins (which, for example, is associated with the phenomenon of incompatibility when transplanting tissues and organs from one animal to another).
The concepts of “protein” and “peptide” are close to each other, but there are also differences between them. Peptides are usually called oligopeptides, i.e. those whose chain contains the largest number of amino acid residues (10-15), and proteins are peptides containing a large number of amino acid residues (up to several thousand) and having a certain compact spatial structure, since they are long the polypeptide chain is an energetically unfavorable state. There are four levels of spatial organization (structure) of proteins. All structures are formed in the channels of the endoplasmic reticulum. When exposed to unfavorable environmental factors (irradiation, elevated temperature, chemicals), protein structures can be destroyed - this occurs denaturation. If this process does not affect the primary structure, it is reversible, and upon completion of the effect the molecule is spontaneously restored. The primary structure is irreducible, since it is formed only on ribosomes with the participation of the most complex mechanism of protein biosynthesis. Depending on the spatial structure, proteins are fibrillar (in the form of fibers) - construction proteins and globular (in the form of a ball) - enzymes, antibodies, some hormones, etc.
The huge variety of proteins provides both the variety of functions they perform and the diversity of organisms.
Functions of proteins:
1) protective (interferon is intensively synthesized in the body during a viral infection);
2) structural (collagen is part of tissues and participates in scar formation);
3) motor (myosin is involved in muscle contraction);
4) spare (egg albumin);
5) transport (hemoglobin in erythrocytes transports nutrients and metabolic products);
6) receptor (receptor proteins ensure that the cell recognizes substances and other cells);
7) regulatory (regulatory proteins determine the activity of genes);
8) hormone proteins are involved in humoral regulation (insulin regulates blood sugar levels);
9) enzyme proteins catalyze all chemical reactions in the body;
10) energy (during decay 1 g protein releases 17 kJ of energy).
Squirrels- high molecular weight organic compounds consisting of α-amino acid residues.
IN protein composition includes carbon, hydrogen, nitrogen, oxygen, sulfur. Some proteins form complexes with other molecules containing phosphorus, iron, zinc and copper.
Proteins have a large molecular weight: egg albumin - 36,000, hemoglobin - 152,000, myosin - 500,000. For comparison: the molecular weight of alcohol is 46, acetic acid - 60, benzene - 78.
Amino acid composition of proteins
Squirrels- non-periodic polymers, the monomers of which are α-amino acids. Typically, 20 types of α-amino acids are called protein monomers, although over 170 of them are found in cells and tissues.
Depending on whether amino acids can be synthesized in the body of humans and other animals, they are distinguished: nonessential amino acids- can be synthesized; essential amino acids- cannot be synthesized. Essential amino acids must be supplied to the body through food. Plants synthesize all types of amino acids.
Depending on the amino acid composition, proteins are: complete- contain the entire set of amino acids; defective- some amino acids are missing in their composition. If proteins consist only of amino acids, they are called simple. If proteins contain, in addition to amino acids, a non-amino acid component (prosthetic group), they are called complex. The prosthetic group can be represented by metals (metalloproteins), carbohydrates (glycoproteins), lipids (lipoproteins), nucleic acids (nucleoproteins).
All amino acids contain: 1) carboxyl group (-COOH), 2) amino group (-NH 2), 3) radical or R-group (the rest of the molecule). The structure of the radical is different for different types of amino acids. Depending on the number of amino groups and carboxyl groups included in the composition of amino acids, they are distinguished: neutral amino acids having one carboxyl group and one amino group; basic amino acids having more than one amino group; acidic amino acids having more than one carboxyl group.
Amino acids are amphoteric compounds, since in solution they can act as both acids and bases. In aqueous solutions, amino acids exist in different ionic forms.
Peptide bond
Peptides- organic substances consisting of amino acid residues connected by peptide bonds.
The formation of peptides occurs as a result of the condensation reaction of amino acids. When the amino group of one amino acid interacts with the carboxyl group of another, a covalent nitrogen-carbon bond occurs between them, which is called peptide. Depending on the number of amino acid residues included in the peptide, there are dipeptides, tripeptides, tetrapeptides etc. The formation of a peptide bond can be repeated many times. This leads to the formation polypeptides. At one end of the peptide there is a free amino group (called the N-terminus), and at the other there is a free carboxyl group (called the C-terminus).

Spatial organization of protein molecules
The performance of certain specific functions by proteins depends on the spatial configuration of their molecules; in addition, it is energetically unfavorable for the cell to keep proteins in an unfolded form, in the form of a chain, therefore polypeptide chains undergo folding, acquiring a certain three-dimensional structure, or conformation. There are 4 levels spatial organization of proteins.
Primary protein structure- the sequence of arrangement of amino acid residues in the polypeptide chain that makes up the protein molecule. The bond between amino acids is a peptide bond.

If a protein molecule consists of only 10 amino acid residues, then the number of theoretically possible variants of protein molecules that differ in the order of alternation of amino acids is 10 20. Having 20 amino acids, you can make even more diverse combinations from them. About ten thousand different proteins have been found in the human body, which differ both from each other and from the proteins of other organisms.
It is the primary structure of the protein molecule that determines the properties of the protein molecules and its spatial configuration. Replacing just one amino acid with another in a polypeptide chain leads to a change in the properties and functions of the protein. For example, replacing the sixth glutamic amino acid in the β-subunit of hemoglobin with valine leads to the fact that the hemoglobin molecule as a whole cannot perform its main function - oxygen transport; In such cases, the person develops a disease called sickle cell anemia.
Secondary structure- ordered folding of the polypeptide chain into a spiral (looks like an extended spring). The turns of the helix are strengthened by hydrogen bonds that arise between carboxyl groups and amino groups. Almost all CO and NH groups take part in the formation of hydrogen bonds. They are weaker than peptide ones, but, repeated many times, impart stability and rigidity to this configuration. At the level of secondary structure, there are proteins: fibroin (silk, spider web), keratin (hair, nails), collagen (tendons).



Tertiary structure- packing of polypeptide chains into globules, resulting from the formation of chemical bonds (hydrogen, ionic, disulfide) and the establishment of hydrophobic interactions between the radicals of amino acid residues. The main role in the formation of the tertiary structure is played by hydrophilic-hydrophobic interactions. In aqueous solutions, hydrophobic radicals tend to hide from water, grouping inside the globule, while hydrophilic radicals, as a result of hydration (interaction with water dipoles), tend to appear on the surface of the molecule. In some proteins, the tertiary structure is stabilized by disulfide covalent bonds formed between the sulfur atoms of two cysteine residues. At the tertiary structure level there are enzymes, antibodies, and some hormones.

Quaternary structure characteristic of complex proteins whose molecules are formed by two or more globules. The subunits are held in the molecule by ionic, hydrophobic, and electrostatic interactions. Sometimes, during the formation of a quaternary structure, disulfide bonds occur between subunits. The most studied protein with a quaternary structure is hemoglobin. It is formed by two α-subunits (141 amino acid residues) and two β-subunits (146 amino acid residues). Associated with each subunit is a heme molecule containing iron.
If for some reason the spatial conformation of proteins deviates from normal, the protein cannot perform its functions. For example, the cause of “mad cow disease” (spongiform encephalopathy) is the abnormal conformation of prions, the surface proteins of nerve cells.
Properties of proteins
The amino acid composition and structure of the protein molecule determine it properties. Proteins combine basic and acidic properties, determined by amino acid radicals: the more acidic amino acids in a protein, the more pronounced its acidic properties. The ability to donate and add H + is determined buffering properties of proteins; One of the most powerful buffers is hemoglobin in red blood cells, which maintains blood pH at a constant level. There are soluble proteins (fibrinogen), and there are insoluble proteins that perform mechanical functions (fibroin, keratin, collagen). There are proteins that are chemically active (enzymes), there are chemically inactive proteins that are resistant to various environmental conditions and those that are extremely unstable.
External factors (heat, ultraviolet radiation, heavy metals and their salts, pH changes, radiation, dehydration)
can cause disruption of the structural organization of the protein molecule. The process of loss of the three-dimensional conformation inherent in a given protein molecule is called denaturation. The cause of denaturation is the breaking of bonds that stabilize a certain protein structure. Initially, the weakest ties are broken, and as conditions become stricter, even stronger ones are broken. Therefore, first the quaternary, then the tertiary and secondary structures are lost. A change in spatial configuration leads to a change in the properties of the protein and, as a result, makes it impossible for the protein to perform its inherent biological functions. If denaturation is not accompanied by destruction of the primary structure, then it may be reversible, in this case, self-recovery of the conformation characteristic of the protein occurs. For example, membrane receptor proteins undergo such denaturation. The process of restoring protein structure after denaturation is called renaturation. If restoration of the spatial configuration of the protein is impossible, then denaturation is called irreversible.
Functions of proteins
| Function | Examples and explanations |
|---|---|
| Construction | Proteins are involved in the formation of cellular and extracellular structures: they are part of cell membranes (lipoproteins, glycoproteins), hair (keratin), tendons (collagen), etc. |
| Transport | The blood protein hemoglobin attaches oxygen and transports it from the lungs to all tissues and organs, and from them transfers carbon dioxide to the lungs; The composition of cell membranes includes special proteins that ensure the active and strictly selective transfer of certain substances and ions from the cell to the external environment and back. |
| Regulatory | Protein hormones take part in the regulation of metabolic processes. For example, the hormone insulin regulates blood glucose levels, promotes glycogen synthesis, and increases the formation of fats from carbohydrates. |
| Protective | In response to the penetration of foreign proteins or microorganisms (antigens) into the body, special proteins are formed - antibodies that can bind and neutralize them. Fibrin, formed from fibrinogen, helps stop bleeding. |
| Motor | The contractile proteins actin and myosin provide muscle contraction in multicellular animals. |
| Signal | Built into the surface membrane of the cell are protein molecules that are capable of changing their tertiary structure in response to environmental factors, thus receiving signals from the external environment and transmitting commands to the cell. |
| Storage | In the body of animals, proteins, as a rule, are not stored, with the exception of egg albumin and milk casein. But thanks to proteins, some substances can be stored in the body; for example, during the breakdown of hemoglobin, iron is not removed from the body, but is stored, forming a complex with the protein ferritin. |
| Energy | When 1 g of protein breaks down into final products, 17.6 kJ is released. First, proteins break down into amino acids, and then into the final products - water, carbon dioxide and ammonia. However, proteins are used as a source of energy only when other sources (carbohydrates and fats) are used up. |
| Catalytic | One of the most important functions of proteins. Provided by proteins - enzymes that accelerate biochemical reactions occurring in cells. For example, ribulose biphosphate carboxylase catalyzes the fixation of CO 2 during photosynthesis. |
Enzymes
Enzymes, or enzymes, are a special class of proteins that are biological catalysts. Thanks to enzymes, biochemical reactions occur at tremendous speed. The speed of enzymatic reactions is tens of thousands of times (and sometimes millions) higher than the speed of reactions occurring with the participation of inorganic catalysts. The substance on which the enzyme acts is called substrate.
Enzymes are globular proteins, structural features enzymes can be divided into two groups: simple and complex. Simple enzymes are simple proteins, i.e. consist only of amino acids. Complex enzymes are complex proteins, i.e. In addition to the protein part, they contain a group of non-protein nature - cofactor. Some enzymes use vitamins as cofactors. The enzyme molecule contains a special part called the active center. Active center- a small section of the enzyme (from three to twelve amino acid residues), where the binding of the substrate or substrates occurs to form an enzyme-substrate complex. Upon completion of the reaction, the enzyme-substrate complex breaks down into the enzyme and the reaction product(s). Some enzymes have (except active) allosteric centers- areas to which enzyme speed regulators are attached ( allosteric enzymes).

Reactions of enzymatic catalysis are characterized by: 1) high efficiency, 2) strict selectivity and direction of action, 3) substrate specificity, 4) fine and precise regulation. The substrate and reaction specificity of enzymatic catalysis reactions are explained by the hypotheses of E. Fischer (1890) and D. Koshland (1959).
E. Fisher (key-lock hypothesis) suggested that the spatial configurations of the active center of the enzyme and the substrate must correspond exactly to each other. The substrate is compared to the “key”, the enzyme to the “lock”.
D. Koshland (hand-glove hypothesis) suggested that the spatial correspondence between the structure of the substrate and the active center of the enzyme is created only at the moment of their interaction with each other. This hypothesis is also called induced correspondence hypothesis.
The rate of enzymatic reactions depends on: 1) temperature, 2) enzyme concentration, 3) substrate concentration, 4) pH. It should be emphasized that since enzymes are proteins, their activity is highest under physiologically normal conditions.
Most enzymes can only work at temperatures between 0 and 40°C. Within these limits, the reaction rate increases approximately 2 times with every 10 °C increase in temperature. At temperatures above 40 °C, the protein undergoes denaturation and enzyme activity decreases. At temperatures close to freezing, enzymes are inactivated.
As the amount of substrate increases, the rate of the enzymatic reaction increases until the number of substrate molecules is equal to the number of enzyme molecules. With a further increase in the amount of substrate, the speed will not increase, since the active centers of the enzyme are saturated. An increase in enzyme concentration leads to increased catalytic activity, since a larger number of substrate molecules undergo transformations per unit time.

For each enzyme, there is an optimal pH value at which it exhibits maximum activity (pepsin - 2.0, salivary amylase - 6.8, pancreatic lipase - 9.0). At higher or lower pH values, enzyme activity decreases. With sudden changes in pH, the enzyme denatures.
The speed of allosteric enzymes is regulated by substances that attach to allosteric centers. If these substances speed up a reaction, they are called activators, if they slow down - inhibitors.
Classification of enzymes
According to the type of chemical transformations they catalyze, enzymes are divided into 6 classes:
- oxireductases(transfer of hydrogen, oxygen or electron atoms from one substance to another - dehydrogenase),
- transferases(transfer of methyl, acyl, phosphate or amino group from one substance to another - transaminase),
- hydrolases(hydrolysis reactions in which two products are formed from the substrate - amylase, lipase),
- lyases(non-hydrolytic addition to the substrate or detachment of a group of atoms from it, in which case C-C, C-N, C-O, C-S bonds can be broken - decarboxylase),
- isomerases(intramolecular rearrangement - isomerase),
- ligases(the connection of two molecules as a result of the formation of C-C, C-N, C-O, C-S bonds - synthetase).
Classes are in turn subdivided into subclasses and subsubclasses. In the current international classification, each enzyme has a specific code, consisting of four numbers separated by dots. The first number is the class, the second is the subclass, the third is the subsubclass, the fourth is the serial number of the enzyme in this subclass, for example, the arginase code is 3.5.3.1.
Go to lectures No. 2"Structure and functions of carbohydrates and lipids"
Go to lectures No. 4"Structure and functions of ATP nucleic acids"