What does type 1 diabetes mean? Diabetes mellitus - symptoms, first signs, causes, treatment, nutrition and complications of diabetes. What it is
Diabetes mellitus is a major medical and social problem throughout the world. This is explained by its wide distribution, the severity of late complications, and the high cost of diagnostic and treatment tools that patients need throughout their lives.
According to experts from the World Health Organization, the total number of patients with all forms of diabetes mellitus today is over 160 million people. Every year, the number of newly diagnosed cases is 6-10% of the total number of patients, thus the number of people suffering from this disease doubles every 10-15 years. Type 1 diabetes is the most severe form of diabetes, accounting for no more than 10% of all cases of the disease. The highest incidence is observed in children aged 10 to 15 years - 40.0 cases per 100 thousand people.
An international expert committee, founded in 1995 with the support of the American Diabetes Association, proposed a new classification, which is accepted in most countries of the world as a recommendation document. The main idea underlying the modern classification of diabetes is a clear identification of the etiological factor in the development of diabetes.
Type 1 diabetes mellitus is a metabolic (metabolic) disease characterized by hyperglycemia, which is based on the destruction of β-cells, leading to an absolute deficiency of insulin. This form of diabetes was previously referred to as insulin-dependent diabetes mellitus or juvenile diabetes mellitus. The destruction of β-cells in most cases among the European population is of an autoimmune nature (with the participation of the cellular and humoral components of the immune system) and is caused by the congenital absence or loss of tolerance to β-cell autoantigens.
Multiple genetic predisposing factors lead to autoimmune destruction of β-cells. The disease has a clear association with the HLA system, with the DQ A1 and DQ B1 genes, as well as DR B1. HLA DR/DQ alleles can be both predisposing and protective.
Type 1 diabetes is often combined with other autoimmune diseases, such as Graves' disease (diffuse toxic goiter), autoimmune thyroiditis, Addison's disease, vitiligo and pernicytic anemia. Type 1 diabetes may be a component of an autoimmune syndrome complex (autoimmune polyglandular syndrome type 1 or 2, “rigid person” syndrome).
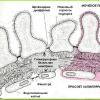
Summarizing the clinical and experimental data obtained to date, we can present the following concept of the pathogenesis of type 1 diabetes. Despite the appearance of an acute onset, type 1 diabetes develops gradually. The latent period can last for several years. Clinical symptoms appear only after 80% of β-cells have been destroyed. An autopsy study of pancreatic tissue from patients with type 1 diabetes reveals the phenomena of insulitis, a specific inflammation characterized by infiltration of islets with lymphocytes and monocytes.
The earliest stages of the preclinical period of type 1 diabetes are characterized by the appearance of clones of autoreactive T lymphocytes that produce cytokines, which leads to the destruction of β-cells. Insulin, glutamate decarboxylase, heat-shock protein 60, and fogrin are currently considered as putative primary autoantigens that, under certain conditions, cause proliferation of cytotoxic T-lymphocytes.
In response to the destruction of β-cells, plasma cells secrete autoantibodies to various β-cell antigens, which are not directly involved in the autoimmune reaction, but indicate the presence of an autoimmune process. These autoantibodies belong to the immunoglobulin G class and are considered as immunological markers of autoimmune damage to β-cells. There are islet cell autoantibodies (ICA - a set of autoantibodies to various cytoplasmic antigens of the β-cell), β-cell-specific autoantibodies to insulin, antibodies to glutamate decarboxylase (GAD), to phosphotyrosine phosphatase (IA-2), and fogrin. Autoantibodies to β-cell antigens are the most important markers of autoimmune destruction of β-cells and they appear in typical type 1 diabetes much earlier than the clinical picture of diabetes develops. Autoantibodies to islet cells appear in the serum 5-12 years before the first clinical manifestations of diabetes mellitus, their titer increases at the late stage of the preclinical period.
There are 6 stages in the development of type 1 diabetes, starting with genetic predisposition and ending with complete destruction of β-cells.
Stage 1 - genetic predisposition - is characterized by the presence or absence of genes associated with type 1 diabetes. The first stage occurs in less than half of genetically identical twins and in 2-5% of siblings. The presence of HLA antigens, especially class II - DR 3, DR 4 and DQ, is of great importance.
Stage 2 - the beginning of the autoimmune process. External factors that can play the role of a trigger in the development of autoimmune damage to β-cells can be: viruses (Coxsackie B virus, rubella, mumps, cytomegalovirus, Epstein-Barr virus), medications, stress factors, nutritional factors (use of infant formula containing animal proteins; products containing nitrosamines). The fact of exposure to various environmental factors can be established in 60% of patients with newly diagnosed type 1 diabetes.
Stage 3 - development of immunological disorders. Specific autoantibodies to various β-cell structures can be detected in the blood: insulin autoantibodies (IAA), ICA, GAD, IA2 and IA2b. In stage 3, there is impaired β-cell function and, as a result of a decrease in β-cell mass, loss of the first phase of insulin secretion, which can be diagnosed by performing an intravenous glucose tolerance test.
Stage 4 - severe immunological disorders - is characterized by impaired glucose tolerance, but there are no clinical signs of diabetes mellitus. When performing an oral glucose tolerance test (OGTT), an increase in glucose levels on an empty stomach and/or 2 hours after the OGTT is detected.
At stage 5, clinical manifestation of the disease is noted, since by this moment the bulk of β-cells (more than 80%) die. Residual low secretion of C-peptide persists for many years and is the most important factor in maintaining metabolic homeostasis. Clinical manifestations of the disease reflect the degree of insulin deficiency.
Stage 6 is characterized by a complete loss of functional activity of β-cells and a decrease in their number. This stage is diagnosed when there is a high level of glycemia, a low level of C-peptide and no response during the exercise test. This stage is called “total” diabetes. Due to the final destruction of β-cells at this stage, a decrease in the titer of antibodies to islet cells or their complete disappearance is sometimes observed.
There is also idiopathic type 1 diabetes mellitus, in which there is a decrease in β-cell function with the development of symptoms of insulinopenia, including ketosis and ketoacidosis, but there are no immunological markers of autoimmune destruction of β-cells. This subtype of diabetes mellitus occurs mainly among patients of African or Asian race. This form of diabetes mellitus has a clear inheritance. The absolute need for replacement therapy in such patients may appear and disappear over time.
As population studies have shown, type 1 diabetes among the adult population is much more common than previously thought. In 60% of cases, type 1 diabetes develops after age 20. The onset of diabetes in adults can have a different clinical picture. The literature describes the asymptomatic development of type 1 diabetes in first- and second-degree relatives of patients with type 1 diabetes with a positive titer of autoantibodies to β-cell antigens, when the diagnosis of diabetes mellitus was made only based on the results of an oral glucose tolerance test.
The classic course of type 1 diabetes with the development of a state of ketoacidosis at the onset of the disease also occurs in adults. The development of type 1 diabetes has been described in all age groups, up to the ninth decade of life.
In typical cases, the onset of type 1 diabetes has pronounced clinical symptoms, reflecting a deficiency of insulin in the body. The main clinical symptoms are: dry mouth, thirst, frequent urination, weight loss. Quite often the onset of the disease is so acute that patients can pinpoint the month, and sometimes even the day, when they first experienced the above symptoms. Rapid, sometimes up to 10-15 kg per month, loss of body weight for no apparent reason is also one of the main symptoms of type 1 diabetes. In some cases, the onset of the disease is preceded by a severe viral infection (influenza, mumps, etc.) or stress. Patients complain of severe weakness and fatigue. Autoimmune diabetes mellitus usually begins in children and adolescents, but can develop at any age.
If symptoms of diabetes mellitus are present, laboratory tests are necessary to confirm the clinical diagnosis. The main biochemical signs of type 1 diabetes are: hyperglycemia (as a rule, a high percentage of sugar in the blood is determined), glucosuria, ketonuria (the presence of acetone in the urine). In severe cases, decompensation of carbohydrate metabolism leads to the development of diabetic ketoacidotic coma.
Diagnostic criteria for diabetes mellitus:
- fasting plasma glucose more than 7.0 mmol/l (126 mg%);
- fasting capillary blood glucose more than 6.1 mmol/l (110 mg%);
- plasma glucose (capillary blood) 2 hours after a meal (or a load of 75 g of glucose) more than 11.1 mmol/l (200 mg%).
Determining the level of C-peptide in serum allows one to assess the functional state of β-cells and, in doubtful cases, to distinguish type 1 diabetes from type 2 diabetes. Measuring C-peptide levels is more informative than insulin levels. In some patients at the onset of type 1 diabetes, a normal basal level of C-peptide may be observed, but there is no increase in it during stimulation tests, which confirms the insufficient secretory ability of β-cells. The main markers confirming the autoimmune destruction of β-cells are autoantibodies to β-cell antigens: autoantibodies to GAD, ICA, insulin. Autoantibodies to islet cells are present in the serum of 80-95% of patients with newly diagnosed type 1 diabetes and in 60-87% of individuals in the preclinical period of the disease.
The progression of β-cell destruction in autoimmune diabetes mellitus (type 1 diabetes) can vary.
In childhood, the loss of β-cells occurs rapidly and by the end of the first year of the disease the residual function fades away. In children and adolescents, the clinical manifestation of the disease usually occurs with symptoms of ketoacidosis. However, in adults there is also a slowly progressive form of type 1 diabetes mellitus, described in the literature as slowly progressive autoimmune diabetes of adults - Latent Autoimmune Diabetes in Adults (LADA).
Slowly progressive autoimmune diabetes of adults (LADA)
This is a special variant of the development of type 1 diabetes mellitus observed in adults. The clinical picture of type 2 diabetes and LADA at the onset of the disease is similar: compensation of carbohydrate metabolism is achieved through diet and/or the use of oral hypoglycemic drugs, but then, during a period that can last from 6 months to 6 years, decompensation of carbohydrate metabolism is observed and insulin need develops. A comprehensive examination of such patients reveals genetic and immunological markers characteristic of type 1 diabetes.
LADA is characterized by the following features:
- age of debut, usually exceeding 25 years;
- clinical picture of type 2 diabetes without obesity;
- initially, satisfactory metabolic control achieved through the use of diet and oral hypoglycemic drugs;
- development of insulin requirements in the period from 6 months to 10 years (on average from 6 months to 6 years);
- presence of markers of type 1 diabetes: low level of C-peptide; the presence of autoantibodies to β-cell antigens (ICA and/or GAD); presence of HLA alleles at high risk of developing type 1 diabetes.
As a rule, patients with LADA do not have a clear clinical picture of the onset of type I diabetes, which is typical for children and adolescents. At onset, LADA is “masked” and initially classified as type 2 diabetes because the process of autoimmune β-cell destruction in adults may be slower than in children. Symptoms of the disease are erased, there is no pronounced polydipsia, polyuria, weight loss and ketoacidosis. Excess body weight also does not exclude the possibility of developing LADA. The function of β-cells fades slowly, sometimes over several years, which prevents the development of ketoacidosis and explains the satisfactory compensation of carbohydrate metabolism when taking PSSP in the first years of the disease. In such cases, type 2 diabetes is mistakenly diagnosed. The gradual nature of the development of the disease leads to the fact that patients seek medical help too late, having time to adapt to the developing decompensation of carbohydrate metabolism. In some cases, patients come to the doctor 1-1.5 years after the manifestation of the disease. In this case, all the signs of a sharp insulin deficiency are revealed: low body weight, high glycemia, lack of effect from PSSP. P. Z. Zimmet (1999) gave the following definition to this subtype of type 1 diabetes: “Autoimmune diabetes that develops in adults may not be clinically different from type 2 diabetes, and is manifested by a slow deterioration of metabolic control with the subsequent development of insulin dependence.” At the same time, the presence in patients of the main immunological markers of type 1 diabetes - autoantibodies to β-cell antigens, along with low basal and stimulated levels of C-peptide, allows a diagnosis of slowly progressing autoimmune diabetes of adults.
Main diagnostic criteria for LADA:
- presence of autoantibodies to GAD and/or ICA;
- low basal and stimulated C-peptide levels;
- presence of HLA alleles at high risk for type 1 diabetes.
The presence of autoantibodies to β-cell antigens in patients with clinical symptoms of type II diabetes at the onset of the disease has a high prognostic value regarding the development of insulin requirement. The results of the UK Prospective Diabetes Study (UKPDS), which examined 3672 patients with an initial diagnosis of type 2 diabetes, showed that antibodies to ICA and GAD have the greatest prognostic value in young patients ( ).
According to P. Zimmet, the prevalence of LADA is about 10-15% among all patients with diabetes mellitus and about 50% of cases occur in type 2 diabetes without obesity.
The results of our study showed that patients aged 30 to 64 years, who at the onset of the disease had a clinical picture of type 2 diabetes without obesity, a significant decrease in body weight (15.5 ± 9.1 kg) and concomitant autoimmune thyroid diseases (TDD) or AIT) represent a group at increased risk of developing LADA. Determination of autoantibodies to GAD, ICA and insulin in this category of patients is necessary for timely diagnosis of LADA. Most often in LADA, antibodies to GAD are detected (according to our data, in 65.1% of patients with LADA), compared with antibodies to ICA (in 23.3% of LADA) and to insulin (in 4.6% of patients). The presence of a combination of antibodies is not typical. The titer of antibodies to GAD in patients with LADA is lower than in patients with type 1 diabetes with the same duration of the disease.
LADA patients represent a high-risk group for developing insulin requirements and require timely administration of insulin therapy. The OGTT results indicate the absence of stimulated insulin secretion in 46% of LADA patients and its decrease in 30.7% of patients already in the first 5 years of the disease. As a result of our study, 41.9% of patients with LADA, whose disease duration was no more than 5 years, were switched to insulin on average 25.2±20.1 months from the onset of the disease. This figure was significantly higher than in the group of patients with type 2 diabetes with the same duration of the disease (14% after 24±21.07 months from the onset of the disease, p< 0,05).
However, patients with LADA represent a heterogeneous group of patients. 53.7% of LADA patients have peripheral insulin resistance, while 30.7% of patients have a combination of insulin resistance and insulin deficiency due to autoimmune damage to β-cells.
When choosing treatment tactics in patients with LADA, insulin secretion and peripheral tissue sensitivity to insulin should be assessed. A basal C-peptide level of less than 1 ng/ml (as determined by radioimmunoassay) indicates insulin deficiency. However, for patients with LADA, the absence of stimulated insulin secretion is more typical, while fasting insulin and C-peptide values are within normal limits (close to the lower limit of normal). The ratio of the maximum insulin concentration (at the 90th minute of the OGTT test) to the initial one is less than 2.8 with low initial values (4.6±0.6 µU/ml), which indicates insufficient stimulated insulin secretion and indicates the need for early administration insulin.
The absence of obesity, decompensation of carbohydrate metabolism when taking PSSP, low basal levels of insulin and C-peptide in LADA patients indicate a high probability of absence of stimulated insulin secretion and the need for insulin administration.
If patients with LADA have a high degree of insulin resistance and insulin hypersecretion in the first years of the disease, it is recommended to prescribe drugs that do not deplete the function of β-cells, but improve the peripheral sensitivity of tissues to insulin, for example biguanides or glitazones (actos, avandia). Such patients are usually overweight and have satisfactory compensation of carbohydrate metabolism, but require further observation. To assess peripheral insulin resistance, the insulin resistance index can be used - Homa-IR = ins0/22.5 eLnglu0 (where ins0 is the fasting insulin level and glu0 is fasting plasma glucose) and/or the index of overall tissue sensitivity to insulin (ISI - insulin sensitivity index, or Matsuda index ), obtained based on the results of the OGTT. With normal glucose tolerance, Homa-IR is 1.21-1.45 points; in patients with type 2 diabetes, the Homa-IR value increases to 6 and even 12 points. The Matsuda index in the group with normal glucose tolerance is 7.3±0.1 UL -1 x ml x mg -1 x ml, and in the presence of insulin resistance its values decrease.
Preserving one's own residual insulin secretion in patients with type 1 diabetes mellitus is very important, since it is noted that in these cases the disease is more stable, and chronic complications develop more slowly and later. The importance of C-peptide in the development of late complications of diabetes mellitus is discussed. It was found that in the experiment, C-peptide improves kidney function and glucose utilization. It was found that infusion of small doses of biosynthetic C-peptide can affect microcirculation in human muscle tissue and renal function.
To determine LADA, more widespread immunological studies are indicated among patients with type 1 diabetes, especially in the absence of obesity and early ineffectiveness of PSSP. The main diagnostic method is the determination of autoantibodies to GAD and to ICA.
A special group of patients who also require close attention and where there is a need to determine autoantibodies to GAD and ICA are women with gestational diabetes mellitus (GDM). It has been established that 2% of women with gestational diabetes mellitus develop type 1 diabetes within 15 years. The etiopathogenetic mechanisms of the development of GDM are very heterogeneous, and for the doctor there is always a dilemma: is GDM the initial manifestation of type 1 or type 2 diabetes. McEvoy et al. published data on the high incidence of autoantibodies to ICA among Native and African-American women in America. According to other data, the prevalence of autoantibodies to ICA and GAD was 2.9 and 5%, respectively, among Finnish women with a history of GDM. Thus, patients with GDM may experience a slow development of insulin-dependent diabetes mellitus, as with LADA diabetes. Screening patients with GDM to determine autoantibodies to GAD and ICA makes it possible to identify patients who require insulin administration, which will make it possible to achieve optimal compensation of carbohydrate metabolism.
Considering the etiopathogenetic mechanisms of LADA development, it becomes obvious the need for insulin therapy in these patients, while early insulin therapy is aimed not only at compensating carbohydrate metabolism, but also allows maintaining basal insulin secretion at a satisfactory level for a long period. The use of sulfonylurea derivatives in LADA patients entails an increased load on β-cells and their faster depletion, while treatment should be aimed at maintaining residual insulin secretion and attenuating the autoimmune destruction of β-cells. In this regard, the use of secretogens in patients with LADA is pathogenetically unjustified.
After clinical manifestation, most patients with a typical clinical picture of type 1 diabetes in a period of 1 to 6 months experience a transient decrease in insulin requirements associated with an improvement in the function of the remaining β-cells. This is the period of clinical remission of the disease, or “honeymoon”. The need for exogenous insulin is significantly reduced (less than 0.4 units/kg body weight); in rare cases, even complete withdrawal of insulin is possible. The development of remission is a distinctive feature of the onset of type 1 diabetes and occurs in 18-62% of cases of newly diagnosed type 1 diabetes. The duration of remission ranges from several months to 3-4 years.
As the disease progresses, the need for exogenously administered insulin increases and averages 0.7-0.8 U/kg body weight. During puberty, the need for insulin can increase significantly - up to 1.0-2.0 U/kg body weight. With increasing duration of the disease due to chronic hyperglycemia, micro- (retinopathy, nephropathy, polyneuropathy) and macrovascular complications of diabetes mellitus (damage to coronary, cerebral and peripheral vessels) develop. The main cause of death is renal failure and complications of atherosclerosis.
Treatment of type 1 diabetes
The goal of treatment for type 1 diabetes is to achieve target levels of glycemia, blood pressure and blood lipids ( ), which can significantly reduce the risk of developing micro- and marcovascular complications and improve the quality of life of patients.
The results of the multicenter randomized Diabetes Control and Complication Trail (DCCT) trial have convincingly shown that good glycemic control reduces the incidence of diabetes complications. Thus, a decrease in glycohemoglobin (HbA1c) from 9 to 7% led to a reduction in the risk of developing diabetic retinopathy by 76%, neuropathy by 60%, and microalbuminuria by 54%.
Treatment of type 1 diabetes includes three main components:
- diet therapy;
- physical exercise;
- insulin therapy;
- training and self-control.
Diet therapy and exercise
When treating type 1 diabetes, foods containing easily digestible carbohydrates (sugar, honey, sweet confectionery, sweet drinks, jam) should be excluded from the daily diet. It is necessary to control the consumption (count bread units) of the following products: grains, potatoes, corn, liquid dairy products, fruits. The daily caloric intake should be covered by 55-60% from carbohydrates, 15-20% from proteins and 20-25% from fats, while the proportion of saturated fatty acids should be no more than 10%.
The physical activity regime should be purely individual. It should be remembered that physical exercise increases tissue sensitivity to insulin, reduces glycemic levels and can lead to the development of hypoglycemia. The risk of hypoglycemia increases during physical activity and for 12-40 hours after prolonged heavy physical activity. Light to moderate exercise lasting no more than 1 hour requires additional intake of easily digestible carbohydrates before and after exercise. With moderate long-term (more than 1 hour) and intense physical activity, adjustment of insulin doses is necessary. It is necessary to measure blood glucose levels before, during and after exercise.
Lifelong insulin replacement therapy is essential for the survival of patients with type 1 diabetes and plays a crucial role in the routine management of this disease. When prescribing insulin, different regimens can be used. Currently, it is customary to distinguish between traditional and intensified insulin therapy regimens.
The main feature of the traditional insulin therapy regimen is the lack of flexible adjustment of the dose of administered insulin to the glycemic level. In this case, self-monitoring of blood glucose is usually absent.
The results of multicenter DCCT convincingly proved the advantage of intensified insulin therapy in compensating carbohydrate metabolism in type 1 diabetes. Intensive insulin therapy includes the following:
- basal-bolus principle of insulin therapy (multiple injections);
- planned number of bread units for each meal (diet liberalization);
- self-monitoring (monitoring blood glucose throughout the day).
For the treatment of type 1 diabetes and the prevention of vascular complications, genetically engineered human insulins are the drugs of choice. Porcine and human semi-synthetic insulins obtained from pork are of lower quality compared to human genetically engineered ones.
Insulin therapy at this stage involves the use of insulins with different durations of action. To create a basic insulin level, intermediate-acting or long-acting insulins are used (approximately 1 unit per hour, which is an average of 24-26 units per day). In order to regulate the level of glycemia after meals, short-acting or ultra-short-acting insulins are used in a dose of 1-2 units per 1 bread unit ( ).
Ultra-short-acting insulins (humalog, novorapid), as well as long-acting insulins (lantus) are insulin analogues. Insulin analogues are specially synthesized polypeptides that have the biological activity of insulin and have a number of specified properties. These are the most promising insulin preparations in terms of intensified insulin therapy. Insulin analogues Humalog (lispro, Lilly), as well as novorapid (aspart, Novo Nordisk) are highly effective in regulating postprandial glycemia. Their use also reduces the risk of hypoglycemia between meals. Lantus (insulin glargine, Aventis) is produced using recombinant DNA technology using a non-pathogenic laboratory strain of Escherichia coli (K12) as a producing organism and differs from human insulin in that the amino acid asparagine from position A21 is replaced by glycine and 2 molecules of arginine are added at C -end of the B-chain. These changes made it possible to obtain a peak-free, constant concentration profile of insulin action over 24 hours/day.
Ready-made mixtures of human insulins of various actions have been created, such as Mixtard (30/70), Insuman Comb (25/75, 30/70), etc., which are stable mixtures of short- and long-acting insulin in specified proportions.
To administer insulin, disposable insulin syringes are used (U-100 for administering insulin with a concentration of 100 U/ml and U-40 for insulin with a concentration of 40 U/ml), syringe pens (Novopen, Humapen, Optipen, Bd-pen, Plivapen) and insulin pumps. All children and adolescents with type 1 diabetes, as well as pregnant women with diabetes, patients with impaired vision and lower limb amputations due to diabetes should be provided with syringe pens.
Achieving target glycemic values is impossible without regular self-monitoring and adjustment of insulin doses. Patients with type 1 diabetes need to independently monitor glycemia daily, several times a day, for which not only glucometers can be used, but also test strips for visual determination of blood sugar (Glucochrome D, Betachek, Suprima Plus).
To reduce the incidence of micro- and macrovascular complications of diabetes, it is important to achieve and maintain normal levels of lipid metabolism and blood pressure.
The target blood pressure level for type 1 diabetes in the absence of proteinuria is BP< 135/85 мм рт. ст., а при наличии протеинурии — более 1 г/сут и при хронической почечной недостаточности — АД < 125/75 мм рт. ст.
The development and progression of cardiovascular diseases largely depends on the level of blood lipids. So, with cholesterol levels above 6.0 mol/l, LDL > 4.0 mmol/l, HDL< 1,0 ммоль/ и триглицеридах выше 2,2 ммоль/л у больных СД 1 типа наблюдается высокий риск развития сердечно-сосудистых осложнений. Терапевтическими целями лечения, определяющими низкий риск развития сердечно-сосудистых осложнений у больных СД 1 типа, являются: общий холестерин < 4,8 ммоль/л, ЛПНП < 3,0 ммоль/л, ЛПВП >1.2 mmol/l, triglycerides< 1,7 ммоль/л.
In the coming decades, research will continue to create new pharmaceutical forms of insulin and means of their administration, which will make replacement therapy as close as possible to the physiological nature of insulin secretion. Research on islet cell transplantation is ongoing. However, a real alternative to allo- or xenotransplantation of cultures or “fresh” islet cells is the development of biotechnological methods: gene therapy, generation of β-cells from stem cells, differentiation of insulin-secreting cells from pancreatic duct cells or pancreatic cells. However, today insulin still remains the main treatment for diabetes.
For questions regarding literature, please contact the editor.
I. V. Kononenko, Candidate of Medical Sciences
O. M. Smirnova,Doctor of Medical Sciences, Professor
Endocrinological Research Center of the Russian Academy of Medical Sciences, Moscow
In the modern world, diabetes mellitus is one of the diseases that is classified as a serious medical and social problem on a global scale, as it has a high prevalence, severe complications, and also requires significant financial costs for diagnostic and therapeutic procedures, which will be necessary for patient all his life. That is why a lot of effort and resources throughout the healthcare sector are aimed at a more in-depth study of the causes and mechanisms of the development of diabetes mellitus, as well as at finding new effective methods for preventing and combating it.
What is type 1 diabetes?
Diabetes mellitus is a chronic disease, the characteristic feature of which is a violation of metabolic processes, accompanied by hyperglycemia (increased blood glucose levels), which occurs as a result of a violation of the production of insulin by the endocrine gland (pancreas), or a violation of its action. Statistics show that the total number of people with diabetes mellitus of all forms in the world currently exceeds 160 million people. New cases of morbidity are recorded so frequently that the number of patients doubles every decade. The most severe form of diabetes mellitus in terms of correction and possible complications is considered to be type 1 diabetes mellitus, the incidence of which ranges from 8-10% of all cases of the disease.
Diabetes mellitus type 1 - a disease of the endocrine system, for which a characteristic feature is an increased concentration of glucose in the blood, which develops due to destructive processes in specific cells of the pancreas that secrete the hormone insulin, resulting in an absolute lack of insulin in the body. A high incidence of type 1 diabetes is observed in children of adolescence and young adulthood - 40 cases per 100,000 people. Previously, this form of diabetes was called insulin-dependent and juvenile diabetes.
There are two forms of diabetes mellitus type 1: autoimmune and idiopathic.
Causes contributing to the development of type 1 diabetes mellitus
Development autoimmune form of diabetes mellitus type 1 It most often begins in childhood, but it can also be diagnosed in older people. In this case, autoantibodies are detected (antibodies produced against the human body’s own antigens) to the structural components of β-cells - specific pancreatic cells that produce insulin, namely, to their surface antigens, insulin, glutamate decarboxylase, etc. They are formed due to congenital or acquired loss of tolerance (insensitivity) to self-antigensβ-cells. As a result of this process, autoimmune destruction of β-cells develops. In children, the process of decay of these cells is rapid, so already a year after the onset of the pathological process, the secretion of insulin in the pancreas completely stops. In the body of adults, the process of cell destruction takes longer, so β-cells can secrete sufficient amounts of insulin over a long period of time, which can prevent the development of such complications of diabetes as ketoacidosis. However, a decrease in insulin secretion is inevitable, and after a certain time its absolute deficiency develops.
Predisposes to autoimmune breakdownpancreatic cells that produce insulin, and a number of genetic factors. Type 1 diabetes mellitus is often diagnosed in combination with autoimmune diseases such as diffuse toxic goiter, autoimmune thyroiditis, Addison's disease, vitiligo, and autoimmune syndrome-complex.
Idiopathic form of diabetes mellitus type 1 is quite rare. In this case, patients do not have immunological and genetic factors for type 1 diabetes mellitus, but there are symptoms confirming absolute insulin deficiency.
The course of type 1 diabetes mellitus
Type 1 diabetes is characterized by a latent period, the duration of which can range from a year to several years. The development of the disease goes through several stages:
Stage 1.Presence of genetic predisposition. If specific antigens of the system are detected in the blood HLA , then the likelihood of developing type 1 diabetes increases significantly.
Stage 2.Suspected trigger factor. It can be agents of an infectious nature - enteroviruses, retroviruses, togaviruses, as well as non-infectious causes - diet, psycho-emotional stress, exposure to chemicals, toxins and poisons, insolation (solar irradiation), radiation, etc.
Stage 3.There are disorders of the immune system - the appearance of autoantibodies to antigensβ-cells, insulin, tyrosine phosphatase - with normal levels of insulin in the blood. In this case, the first phase of insulin production is absent.
Stage 4.It is characterized by serious immune disruptions, namely, insulin secretion rapidly decreases due to the development of insulitis (inflammation in the islets of Langerhans of the pancreas, containing cells that produce insulin), glucose resistance is impaired, while blood sugar levels remain within normal limits.
Stage 5.It is characterized by pronounced clinical manifestations, since three quartersβ-cells are destroyed at this point. Only residual secretion of C-peptide is preserved.
Stage 6.Total death of β-cells. C-peptide is not detected, antibody titers decrease. This stage is otherwise called total diabetes. The course of diabetes mellitus becomes uncontrollable, which threatens the development of severe complications - disseminated intravascular coagulation, edema of the cerebral cortex and the development of diabetic coma.
How does type 1 diabetes manifest?
Since clinical signs appear when most of the β-cells of the pancreas are destroyed, the onset of the disease is always acute and may appear for the first time severe acidosis or diabetic coma. In children and adolescents, the onset of the disease is characterized by signs of ketoacidosis. Sometimes patients can clearly name the day when they noticed the signs of the disease. Sometimes the onset of the disease may be preceded by a severe viral infection (influenza, mumps, rubella).
Patients may complain of dry mouth and a feeling of thirst caused by excessive excretion of fluid from the body by the kidneys, frequent urination, increased appetite along with an impressive loss of body weight (up to 10-15 kg per month), general weakness, and fatigue. In addition, patients may complain of itching, pustular processes on the skin and nails, and blurred vision. On the sexual side, patients note a decrease in sexual desire and potency. In the oral cavity, signs of periodontal disease, alveolar pyorrhea, gingivitis, and stomatitis may be detected. carious lesions of teeth.
When examining patients with type 1 diabetes, an increase in the concentration of sugar in the blood and its presence in the urine is detected. In the stage of decompensation, experts note dryness of the skin of patients, their mucous membranes, tongue, decreased turgor of subcutaneous fat, redness of the cheeks, forehead and chin due to dilation of the skin capillaries of the face. If the decompensation process is prolonged, patients may develop complications such as diabetic ophthalmopathy, nephropathy, peripheral neuropathy, diabetic osteoarthropathy, etc. Girls may develop infertility, and children may experience noticeable impairment and retardation in growth and physical development.
Diagnostic criteria for type 1 diabetes mellitus
If, along with clinical signs, there is an increased concentration of glucose in the blood (more than 11.1 mmol/l) at any time of the day, then we can talk about diabetes mellitus.
Experts from the World Health Organization have developed a number of criteria that are used to diagnose diabetes mellitus. First of all, this is determining the level of glucose in the blood on an empty stomach, that is, when at least 8 hours have passed since the last meal. It is also necessary to determine the level of glucose in the blood randomly, namely, at any time within 24 hours, regardless of the time of food consumption.
In order to assess at what stage of diabetes the patient is, the following laboratory tests are necessary:
General analysis of urine and blood;
The concentration of glucose in the blood on an empty stomach, and then a couple of hours after eating;
Determination of the level of glycosylated hemoglobin;
Level of ketone bodies and glucose in daily urine;
Blood chemistry;
Urinalysis according to Nechiporenko.
For the purpose of differential diagnosis of type 1 diabetes mellitus, an analysis is carried out for the content of immunological and genetic markers and the level of C-peptide.
In addition, patients undergo a number of mandatory instrumental studies - electrocardiography, chest x-ray and ophthalmoscopy.
Despite the fact that the clinical picture of insulin-dependent and non-insulin-dependent diabetes mellitus has many similarities, the differential diagnosis between them is based on a number of differences. If type 1 diabetes mellitus is characterized by a decrease in the body weight of patients, then type 2 diabetes mellitus is characterized by weight gain. Type 1 diabetes mellitus begins acutely, unlike type 2 diabetes mellitus, which is characterized by a slow increase in symptoms. Type 2 diabetes mellitus is more often diagnosed in adults and older people (over 45 years of age), and type 1 diabetes mellitus is more often diagnosed in children and young people. In laboratory studies, antibodies to β-cell antigens are detected only in insulin-dependent diabetes.
If a patient is diagnosed with type 1 diabetes for the first time, he must be hospitalized in order to select an insulin treatment regimen, learn how to independently monitor blood glucose levels, develop a diet and work regimen. In addition, patients in a precomatous and comatose state, with diabetic ketoacidosis, with an increase in angiopathy, with the addition of infections, as well as if any surgical intervention is necessary, are subject to hospitalization.
Treatment of type 1 diabetes
The main goal of treating patients with type 1 diabetes is to preserve their life, as well as improve its quality. For this purpose, preventive measures are taken to prevent the development of acute and chronic complications and correction of concomitant pathologies.
Treatment of type 1 diabetes mellitus involves a complex of measures, including insulin therapy, which is currently the only method for correcting absolute insulin deficiency. For these purposes, our country uses analogues of human insulin or insulin obtained by genetic engineering. Insulin replacement therapy can be carried out according to the traditional regimen, when a certain level of insulin is administered subcutaneously without constantly adapting the dose to the glycemic level. Intensive insulin therapy has great advantages, which includes multiple insulin injections, diet correction by counting bread units and monitoring glucose levels throughout the day.
The next point in the diabetes treatment regimen is the development of a special nutrition program that will normalize body weight and help maintain blood glucose levels within normal limits. Food for patients with diabetes should be low in calories, not contain refined carbohydrates (confectionery, sweet drinks, jams), and meal times should be strictly observed. It is necessary to exclude canned food, smoked meats, and foods high in fat (sour cream, mayonnaise, nuts) from the diet. The ratio of the main energy components in the diet is usually equated to physiological, and it is 3:1:1.
Physical activity for patients with type 1 diabetes should be moderate and selected individually, based on the severity of the disease. The best form of physical activity is walking. However, it should be remembered that shoes should be selected in such a way as to prevent the formation of corns and calluses, which can become the beginning of a dangerous complication of diabetes - diabetic foot.
The outcome of diabetes treatment is directly related to the active participation of the patient himself, who must be trained by medical personnel in methods of self-monitoring of blood glucose levels using glucometers and test strips, because he needs to carry out this manipulation at least 3-4 times a day. In addition, the patient must assess his condition, control his diet and amount of physical activity, and also regularly visit the attending physician, who, in addition to talking with the patient, must examine his legs and measure blood pressure. Once a year, a patient with type 1 diabetes must undergo all the necessary tests (biochemical blood test, general blood and urine test, determination of the level of glycosylated hemoglobin), undergo an examination by an ophthalmologist and neurologist, and have a chest x-ray.
Preventing the development of type 1 diabetes mellitus
The development of type 1 diabetes mellitus in people with a high genetic predisposition can be prevented by preventing intrauterine viral infections, as well as contracting viral infections in childhood and adolescence. You should not include in the diet of children predisposed to the disease, nutritional formulas containing gluten, foods with preservatives and dyes that can cause an autoimmune reaction against insulin-producing cells of the pancreas.
Complications of diabetes
The main reason for the development of complications of diabetes mellitus is vascular damage due to prolonged decompensation of diabetes mellitus (prolonged hyperglycemia - high blood sugar). First of all, microcirculation suffers, that is, the blood supply to the smallest vessels is disrupted
Treatment of diabetes
Diabetes mellitus is a group of metabolic diseases characterized by high levels of glucose (“sugar”) in the blood
Types of diabetes
Currently, there are two main types of diabetes mellitus, differing in the cause and mechanism of occurrence, as well as in the principles of treatment
Diet for diabetes
Numerous studies around the world are focused on finding effective treatments for diabetes. However, we should not forget that in addition to drug therapy, recommendations for lifestyle changes are no less important.
Gestational diabetes mellitus during pregnancy
Gestational diabetes mellitus can develop during pregnancy (in approximately 4% of cases). It is based on a decrease in the ability to absorb glucose
Hypoglycemia
Hypoglycemia is a pathological condition characterized by a decrease in plasma glucose concentration below 2.8 mmol/l, occurring with certain clinical symptoms, or less than 2.2 mmol/l, regardless of the presence or absence of clinical signs
Coma with diabetes mellitus
Information about the most dangerous complication of diabetes mellitus, which requires emergency medical care, is coma. The types of comas in diabetes mellitus, their specific symptoms, and treatment tactics are described.
Autoimmune polyglandular syndrome
Autoimmune polyglandular syndrome is a group of endocrinopathies, which is characterized by the involvement of several endocrine glands in the pathological process as a result of their autoimmune damage
Diabetic foot syndrome is one of the complications of diabetes mellitus, along with diabetic ophthalmopathy, nephropathy, etc., which is a pathological condition resulting from damage to the peripheral nervous system, arterial and microvasculature, manifested by purulent-necrotic, ulcerative processes and damage to the bones and joints of the foot
About diabetes
Diabetes mellitus is a term that unites endocrine diseases, the characteristic feature of which is the insufficiency of the action of the hormone insulin. The main symptom of diabetes mellitus is the development of hyperglycemia - an increase in the concentration of glucose in the blood, which is persistent.
Diabetes symptoms
The effectiveness of diabetes treatment directly depends on the time of detection of this disease. In type 2 diabetes mellitus, the disease can cause only mild complaints for a long time, to which the patient may not pay attention. Symptoms of diabetes can be subtle, making diagnosis difficult. The earlier the correct diagnosis is made and treatment is started, the lower the risk of developing diabetes complications.
Very often, patients under 18 years of age come to see specialists at the Northwestern Endocrinology Center. For them, the center has special doctors - pediatric endocrinologists.
Type 1 diabetes mellitus is an autoimmune endocrine disease, the main diagnostic criterion of which is chronic hyperglycemia, due to an absolute lack of insulin production by beta cells of the pancreas.
Insulin is a protein hormone that helps glucose move from the blood into cells. Without it, glucose is not absorbed and remains in the blood in high concentration. A high level of glucose in the blood does not provide energy value, and with prolonged hyperglycemia, damage to blood vessels and nerve fibers begins. At the same time, the cells are “starved” of energy, they do not have enough glucose to carry out metabolic processes, then they begin to extract energy from fats, and then from proteins. All this leads to many consequences, which we will discuss below.
The term “glycemia” means blood sugar level.
Hyperglycemia is elevated blood sugar levels.
Hypoglycemia – blood sugar is below normal.
A glucometer is a device for independently determining capillary blood sugar. Blood is drawn using a scarifier (disposable needles included in the kit), a drop of blood is applied to a test strip and inserted into the device. Numbers are displayed on the screen that reflect the current blood sugar level.
Causes of type 1 diabetes
The reasons are genetic and hereditary predisposition is of primary importance.
Classification of type 1 diabetes mellitus
1. By compensation
Compensated diabetes is a state of diabetes in which the levels of carbohydrate metabolism are close to those of a healthy person.
Subcompensation. There may be short-term episodes of hyperglycemia or hypoglycemia, without significant impairment of vital functions.
Decompensation. Blood sugar fluctuates widely, with hypoglycemic and hyperglycemic states, up to the development of precoma and coma. Acetone (ketone bodies) appears in the urine.
2. According to the presence of complications
Uncomplicated (initial course or ideally compensated diabetes, which does not have complications, which are described below);
- complicated (there are vascular complications and/or neuropathies)
3. By origin
Autoimmune (antibodies to one’s own cells have been detected);
- idiopathic (the cause has not been identified).
This classification has only scientific significance, since it does not influence treatment tactics.
Symptoms of type 1 diabetes:
1. Thirst (with elevated blood sugar, the body requires “dilution” of the blood, reducing glycemia, this is achieved by drinking plenty of fluids, this is called polydipsia).
2. Copious and frequent urination, urination at night (intake of large amounts of liquid, as well as high levels of glucose in the urine contribute to urination in large, unusual volumes, this is called polyuria).
3. Increased appetite (don’t forget that the body’s cells are starving and therefore signal their needs).
4. Weight loss (cells, not receiving carbohydrates for energy, begin to feed on fats and proteins, so there is no material left for the construction and renewal of tissues, a person loses weight with increased appetite and thirst).
5. The skin and mucous membranes are dry, and there are often complaints that the mouth “dries out.”
6. General condition with reduced performance, weakness, fatigue, muscle pain and headaches (also due to energy starvation of all cells).
7. Attacks of sweating, itching of the skin (in women, itching in the perineum may often be the first to appear).
8. Low resistance to infection (exacerbation of chronic diseases, such as chronic tonsillitis, the appearance of thrush, susceptibility to acute viral infections).
9. Nausea, vomiting, abdominal pain in the epigastric region (under the stomach).
10. In the long-term period, the appearance of complications: decreased vision, impaired renal function, impaired nutrition and blood supply to the lower extremities, impaired motor and sensory innervation of the extremities, as well as the formation of autonomic polyneuropathy.
Diagnostics:

Angiopathy in diabetes
As already mentioned, a high concentration of blood glucose damages the vascular wall, which leads to the development of microangiopathy (damage to small vessels) and macroangiopathy (damage to large vessels).
To microangiopathies include: retinal retinopathy (damage to small vessels of the eyes), nephropathy (damage to the vascular apparatus of the kidneys) and lesions of small vessels of other organs. Clinical signs of microangiopathy appear approximately between 10 and 15 years of type 1 diabetes, but there may be deviations from statistics. If diabetes is well compensated and additional treatment is carried out in a timely manner, then the development of this complication can be “postponed” indefinitely. There are also cases of very early development of microangiopathy, already 2–3 years from the onset of the disease.
In young patients, vascular damage is “purely diabetic”, and in the older generation it is combined with vascular atherosclerosis, which worsens the prognosis and course of the disease.
Morphologically, microangiopathy is a multiple lesion of small vessels in all organs and tissues. The vascular wall thickens, and hyaline deposits appear on it (a protein substance of high density and resistant to various influences). Because of this, the vessels lose their normal permeability and flexibility, nutrients and oxygen have difficulty penetrating the tissues, the tissues are depleted and suffer from a lack of oxygen and nutrition. In addition, the affected vessels become more vulnerable and fragile. As has already been said, many organs are affected, but the most clinically significant is damage to the kidneys and retina.
Prevention of type 1 diabetes
In the case of type 1 diabetes mellitus, the patient’s task is to prevent complications. Regular consultations with an endocrinologist, as well as participation in Diabetes Schools, will help you with this. The Diabetes School is an awareness-raising activity carried out by doctors of various specialties. Endocrinologists, surgeons and therapists teach patients to count bread units, self-monitor blood sugar, recognize worsening conditions and provide self- and mutual assistance, care for their feet (this is extremely important in the development of angiopathy and neuropathy) and other useful skills.
Type 1 diabetes is a disease that becomes a way of life. It changes your usual routine, but does not interfere with your success and life plans. You are not limited in your professional activities, freedom of movement and desire to have children. Many famous people live with diabetes, including Sharon Stone, Halle Berry, hockey player Bobby Clarke and many others. The key to success is self-control and timely consultation with a doctor. Take care of yourself and be healthy!
General practitioner Petrova A.V.
Diabetes mellitus is currently one of the most common diseases, mainly due to the fact that it affects more and more young people. There is, however, it is type 1 diabetes that manifests itself in patients under the age of 30, causing certain symptoms and requiring insulin.
The causes of type 1 diabetes are now precisely known. Genetic tendencies are to a certain extent responsible for its appearance. Especially when close relatives have diabetes. It will not necessarily be the same type - often the disease itself is inherited, which manifests itself in various forms.
Type 1 diabetes is caused primarily by past infections, mostly viral. When they occur or as a result of improper treatment, damage occurs to pancreatic cells called islets of Langerhans or beta cells. As a result, the gland begins to malfunction, disrupting the production of insulin.
This type of diabetes was called juvenile diabetes for a long time, because. most diseases occur between 20 and 30 years of age. The first symptoms can appear in early adolescence, as well as before the age of 18 - this is almost half of the number of diagnosed cases.
Type 1 diabetes mellitus is a disease of young people, it is also called juvenile diabetes. People under the age of 35 are susceptible to the disease. There are causes of juvenile diabetes 1a, presumably of a viral nature, which appear only in childhood, and causes of juvenile diabetes 1b (the most common) - antibodies to insulin cells are detected, there is a decrease or cessation of insulin production by the pancreas. This type accounts for 1.5–2% of all cases of diabetes.
Juvenile diabetes is a disease with a hereditary predisposition, but the contribution of the genotype to the development of the disease is small. It occurs in children with a sick mother with a probability of 1-2%, father - 3-6%, brother or sister - 6%. Having type 2 diabetes in first-degree family members also increases the risk of developing type 1 diabetes.
If a person with a hereditary predisposition gets a virus into the body, the infectious disease will provoke the development of antibodies to beta cells. As a result, the cells that produce insulin will die. But the “insidiousness” of diabetes is that signs of the disease will not appear immediately - more than 80% of β-cells must first be destroyed, which can happen over several months or several years. As a result, many patients immediately experience absolute insulin deficiency.
As a rule, the disease develops according to the following scenario:
- The presence of a genetic predisposition to diabetes.
- Destruction of β-cells (cells of the islets of Langerhans) of the pancreas. Cell death can be of an autoimmune nature or begin under the influence of environmental factors, for example, after viral infections enter the body. Such agents may be cytomegalovirus, rubella, measles, Coxsackie B virus, chickenpox, and mumps viruses. Toxic substances are also known that selectively affect beta cells and induce an autoimmune reaction.
- Psycho-emotional stress. There are cases of sudden onset diabetes after severe stress. Stressful situations are provocateurs for the exacerbation of various chronic diseases and the effects of viruses.
- An inflammatory reaction in the pancreatic islets called “insulitis.”
- Transformation of β-cells by the immune system because they are perceived as foreign.
- Rejection of pancreatic islets, cytotoxic antibodies appear.
- Destruction of β-cells and the appearance of obvious signs of diabetes.
Type 1 diabetes mellitus has severe symptoms and is quite acute, characterized by a progressive deterioration of the patient's condition in the absence of treatment. Typically, patients can accurately name the period of onset of the first symptoms. It is characterized by thirst, frequent and copious urination, sometimes more than 6 liters per day, dry mouth, general weakness, fatigue, itchy skin, itching in the perineum, insatiable hunger and weight loss.
Quite common symptoms are also considered irritability, pain in the heart, in the calf muscles, headache, etc. The examination reveals sugar in the urine, increased blood glucose and insulin deficiency. Moreover, the level of insulin in plasma can be so low that it is not even detectable.
In clinically significant diabetes, fasting blood sugar is >120 mg/dL or >6.7 mmol/L, and blood sugar 2 hours after a main meal is >180 mg/dL or >10 mmol/L. There is a rapid deterioration in health and severe dehydration. If insulin drugs are not prescribed in time, the patient may develop a diabetic coma.
The disease is dangerous due to the development of complications: stroke, heart attack, eye damage up to blindness, with the development of renal failure, resulting in gangrene and loss of limb, muscle atrophy, osteoporosis, etc.
Insulin therapy is necessary at the first symptoms of type 1 diabetes. It is worth noting that there are cases of complete normalization of metabolism with the help of insulin preparations. That is, type 1 diabetes mellitus can be remitted with timely detection and administration of insulin. However, even in such cases, complete recovery is impossible.
Currently, diabetes mellitus is an incurable disease. The main method of its treatment is only the regular injection of insulin into the body. If diabetes has already developed, then it is impossible to restore β-cells. They are trying to transplant the pancreas and cells that produce insulin, but so far without success.
Unfortunately, there is not yet a form of insulin that would not be destroyed by gastric juice once it enters the stomach through the mouth. Therefore, insulin therapy is carried out by injection or by sewing up an insulin pump. In addition to traditional insulin syringes, there are pen-style injection devices that make insulin injections easy and convenient.
In this case, the following types of insulin preparations are used:
- interim validity period
- fast-acting
- long-lasting
The choice of the optimal drug, as well as the selection of dosage and number of injections, should be made by an endocrinologist.
Most patients receiving insulin medications control their condition by self-monitoring their blood sugar levels. This is important because the main thing in treating type 1 diabetes is to strive for constant blood glucose concentrations. To maintain the glycemic level at a certain level, you must follow some rules when selecting the dosage of insulin:
It is possible to achieve normoglycemia not only by varying insulin doses, but also by constantly keeping track of calories consumed. Based on your ideal body weight, you need to calculate your protein, fat and carbohydrate intake and create a balanced diet.
There are certain rules that a patient with this type of diabetes must follow:
Sugar, jam, sweets and other quickly absorbed carbohydrates are completely excluded, as they provoke a sharp jump in blood glucose levels. They are recommended to be consumed only during an attack of hypoglycemia, in combination with “complex” carbohydrates and fiber.
“Complex” carbohydrates are found in cereals, beans, potatoes and other vegetables. They take longer to digest, which is very beneficial for patients with type 1 diabetes. Sufficient inclusion of vegetables, fruits and berries in the diet is beneficial, as they contain vitamins and microelements, are rich in dietary fiber and ensure normal metabolism in the body.
Attention!
But you should keep in mind that some fruits and berries (prunes, strawberries, etc.) contain a lot of carbohydrates, so they can be consumed only taking into account the daily amount of carbohydrates in the diet.
The food industry produces special varieties of bread, cookies, biscuits, and cakes that contain significantly less easily digestible carbohydrates than usual. To satisfy taste needs, and also partly for medicinal purposes, the inclusion of various sugar substitutes is recommended.
The consumption of alcoholic beverages should be sharply limited or stopped, since alcohol is a high-calorie drink, and in addition has an adverse effect on the functions of all organs and systems (primarily the nervous system).
To normalize blood glucose levels, it is important not only to follow a diet, but also to lead an active lifestyle. Any physical activity improves blood circulation and lowers blood sugar levels:
- during exercise, the sensitivity of body tissues to insulin and the rate of its absorption increase
- glucose consumption increases without additional insulin doses
- With regular training, normoglycemia stabilizes much faster
Physical exercise greatly affects carbohydrate metabolism, so it is important to remember that during training the body actively uses glycogen reserves, so hypoglycemia may occur after exercise. You cannot exercise if you are feeling unwell. It is important to have “simple” carbohydrates with you, for example, a couple of candies.
In order for muscle cells to absorb glucose, there must be enough insulin in the blood. You should start exercising when your blood sugar level is not lower than 5 mmol/l and not higher than 15 mmol/l. It is best to exercise with a trainer or with friends who have knowledge about diabetes and hypoglycemia care.
Type 1 diabetes requires regular and dosed exercise. Sudden intense exercise causes an imbalance in blood glucose levels. A brisk walk, jogging, active housework, or going to a disco can be considered physical activity. The ideal physical activity is walking.
The optimal mode of physical activity is exercise 5 times a week for 30 minutes. The intensity of the exercise should be such that the patient’s heart rate reaches up to 65% of the maximum. The maximum heart rate is calculated individually using the formula: 220 minus age. When walking, you should not forget about the requirements for shoes, which should not injure your feet. If you have diabetic foot syndrome, you should pay special attention to foot care after exercise.
Source: http://insulat.ru/diabet/diabet_1_tipa
Diabetes mellitus type 1
In the modern world, diabetes mellitus is one of the diseases that is classified as a serious medical and social problem on a global scale, as it has a high prevalence, severe complications, and also requires significant financial costs for diagnostic and therapeutic procedures, which will be necessary for patient all his life.
That is why a lot of effort and resources throughout the healthcare sector are aimed at a more in-depth study of the causes and mechanisms of the development of diabetes mellitus, as well as to find new effective ones to combat it.
What is type 1 diabetes?
Diabetes mellitus is a chronic disease, the characteristic feature of which is a violation of metabolic processes, accompanied by (increased blood glucose levels), which occurs as a result of a violation of the production of insulin by the endocrine gland (pancreas), or a violation of its action.
Statistics show that the total number of people with diabetes mellitus of all forms in the world currently exceeds 160 million people. New cases of morbidity are recorded so frequently that the number of patients doubles every decade. The most severe form of diabetes mellitus in terms of correction and possible complications is considered to be type 1 diabetes mellitus, the incidence of which ranges from 8-10% of all cases of the disease.
Type 1 diabetes mellitus is a disease of the endocrine system, for which a characteristic feature is an increased concentration of glucose in the blood, which develops due to destructive processes in specific pancreatic cells that secrete the hormone insulin, resulting in an absolute lack of insulin in the body. A high incidence of type 1 diabetes is observed in children of adolescence and young adulthood - 40 cases per 100,000 people. Previously, this form of diabetes was called insulin-dependent and juvenile diabetes.
There are two forms of type 1 diabetes: autoimmune and idiopathic.
Reasons promoting development
The development of the autoimmune form of type 1 diabetes mellitus most often begins in childhood, but it can also be diagnosed in older people. In this case, autoantibodies are detected (antibodies produced against the human body’s own antigens) to the structural components of β-cells - specific pancreatic cells that produce insulin, namely, to their surface antigens, insulin, glutamate decarboxylase, etc.
They are formed due to congenital or acquired loss of tolerance (insensitivity) to self-antigens of β-cells. As a result of this process, autoimmune destruction of β-cells develops. In children, the process of decay of these cells is rapid, so already a year after the onset of the pathological process, the secretion of insulin in the pancreas completely stops.
In the body of adults, the process of cell destruction takes longer, so β-cells can secrete sufficient amounts of insulin over a long period of time, which can prevent the development of such complications of diabetes as ketoacidosis. However, a decrease in insulin secretion is inevitable, and after a certain time its absolute deficiency develops.
A number of genetic factors also predispose to autoimmune destruction of pancreatic cells that produce insulin. Type 1 diabetes mellitus is often diagnosed in combination with autoimmune diseases such as diffuse toxic goiter, autoimmune thyroiditis, Addison's disease, vitiligo, and autoimmune syndrome-complex.
The idiopathic form of type 1 diabetes is quite rare. In this case, patients do not have immunological and genetic factors for type 1 diabetes mellitus, but there are symptoms confirming absolute insulin deficiency.
Flow
Type 1 diabetes is characterized by a latent period, the duration of which can range from a year to several years. The development of the disease goes through several stages:
- Stage 1. Presence of genetic predisposition. If specific antigens of the HLA system are detected in the blood, then the likelihood of developing type 1 diabetes increases significantly.
- Stage 2. Suspected trigger factor. It can be agents of an infectious nature - enteroviruses, retroviruses, togaviruses, as well as non-infectious causes - diet, psycho-emotional stress, exposure to chemicals, toxins and poisons, insolation (solar irradiation), radiation, etc.
- Stage 3. There are disorders of the immune system - the appearance of autoantibodies to β-cell antigens, insulin, tyrosine phosphatase - with normal levels of insulin in the blood. In this case, the first phase of insulin production is absent.
- Stage 4. It is characterized by serious immune disruptions, namely, insulin secretion rapidly decreases due to the development of insulitis (inflammation in the islets of Langerhans of the pancreas, containing cells that produce insulin), glucose resistance is impaired, while blood sugar levels remain within normal limits.
- Stage 5. Pronounced clinical manifestations are typical for it, since three quarters of the β-cells are destroyed by this point. Only residual secretion of C-peptide is preserved.
- Stage 6. Total death of β-cells. C-peptide is not detected, antibody titers decrease. This stage is otherwise called total diabetes. The course of diabetes mellitus becomes uncontrollable, which threatens the development of severe complications - disseminated intravascular coagulation, edema of the cerebral cortex and the development of diabetic coma.
How does it manifest?
Because clinical signs appear when most of the β-cells of the pancreas are destroyed, the onset of the disease is always acute and may first manifest as severe acidosis or diabetic coma. In children and adolescents, the onset of the disease is characterized by symptoms. Sometimes patients can clearly name the day when they noticed the signs of the disease. Sometimes the onset of the disease may be preceded by a severe viral infection (influenza, mumps, rubella).
Patients may complain of dry mouth and a feeling of thirst caused by excessive excretion of fluid from the body by the kidneys, frequent urination, increased appetite along with an impressive loss of body weight (up to 10-15 kg per month), general weakness, and fatigue.
In addition, patients may complain of itching, pustular processes on the skin and nails, and blurred vision. On the sexual side, patients note a decrease in sexual desire and potency. In the oral cavity, signs of periodontal disease, alveolar pyorrhea, gingivitis, and stomatitis may be detected. carious lesions of teeth.
When examining patients with type 1 diabetes, an increase in the concentration of sugar in the blood and its presence in the urine is detected. In the stage of decompensation, experts note dryness of the skin of patients, their mucous membranes, tongue, decreased turgor of subcutaneous fat, redness of the cheeks, forehead and chin due to dilation of the skin capillaries of the face.
If the decompensation process is prolonged, patients may develop complications such as diabetic ophthalmopathy, nephropathy, peripheral neuropathy, diabetic osteoarthropathy, etc. Girls may develop infertility, and children may experience noticeable impairment and retardation in growth and physical development.
Diagnostic criteria
If, along with clinical signs, there is an increased concentration of glucose in the blood (more than 11.1 mmol/l) at any time of the day, then we can talk about diabetes mellitus.
Experts from the World Health Organization have developed a number of criteria that are used to diagnose diabetes mellitus. First of all, this is determining the level of glucose in the blood on an empty stomach, that is, when at least 8 hours have passed since the last meal. It is also necessary to determine the level of glucose in the blood randomly, namely, at any time within 24 hours, regardless of the time of food consumption.
In order to assess at what stage of diabetes the patient is, the following laboratory tests are necessary:
- general analysis of urine and blood;
- the concentration of glucose in the blood on an empty stomach, and then a couple of hours after eating;
- determination of the level of glycosylated hemoglobin;
- level of ketone bodies and glucose in daily urine;
- blood chemistry;
- urine analysis according to Nechiporenko.
For the purpose of differential diagnosis of type 1 diabetes mellitus, an analysis is carried out for the content of immunological and genetic markers and the level of C-peptide. In addition, patients undergo a number of mandatory instrumental studies - electrocardiography, chest x-ray and ophthalmoscopy.
Attention!
Despite the fact that the clinical picture of insulin-dependent and non-insulin-dependent diabetes mellitus has many similarities, the differential diagnosis between them is based on a number of differences. If type 1 diabetes mellitus is characterized by a decrease in the body weight of patients, then type 2 diabetes mellitus is characterized by weight gain.
Type 1 diabetes mellitus begins acutely, unlike type 2 diabetes mellitus, which is characterized by a slow increase in symptoms. Type 2 diabetes mellitus is more often diagnosed in adults and older people (over 45 years of age), and type 1 diabetes mellitus is more often diagnosed in children and young people. In laboratory studies, antibodies to β-cell antigens are detected only in insulin-dependent diabetes.
If a patient is diagnosed with type 1 diabetes for the first time, he must be hospitalized in order to select an insulin treatment regimen, learn how to independently monitor blood glucose levels, develop a diet and work regimen.
In addition, patients in a precomatous and comatose state, with diabetic ketoacidosis, with an increase in angiopathy, with the addition of infections, as well as if any surgical intervention is necessary, are subject to hospitalization.
Treatment
The main goal of treating patients with type 1 diabetes is to preserve their life, as well as improve its quality. For this purpose, preventive measures are taken to prevent the development of acute and chronic complications and correction of concomitant pathologies.
Treatment of type 1 diabetes mellitus involves a complex of measures, which includes insulin therapy, which is currently the only method for correcting absolute insulin deficiency. For these purposes, our country uses analogues of human insulin or insulin obtained by genetic engineering.
Insulin replacement therapy can be carried out according to the traditional regimen, when a certain level of insulin is administered subcutaneously without constantly adapting the dose to the glycemic level. Intensive insulin therapy has great advantages, which includes multiple insulin injections, diet correction by counting bread units and monitoring glucose levels throughout the day.
The next point in the diabetes treatment regimen is the development of a special nutrition program that will normalize body weight and help maintain blood glucose levels within normal limits. Food for patients with diabetes should be low in calories, not contain refined carbohydrates (confectionery, sweet drinks, jams), and meal times should be strictly observed.
It is necessary to exclude canned food, smoked meats, and foods high in fat (sour cream, mayonnaise, nuts) from the diet. The ratio of the main energy components in the diet is usually equated to physiological, and it is 3:1:1.
Physical activity for patients with type 1 diabetes should be moderate and selected individually, based on the severity of the disease. The best form of physical activity is walking. However, it should be remembered that shoes should be selected in such a way as to prevent the formation of corns and calluses, which can become the beginning of a dangerous complication of diabetes mellitus - diabetic foot.
The outcome of diabetes treatment is directly related to the active participation of the patient himself, who must be trained by medical personnel in methods of self-monitoring of blood glucose levels using test strips, because he needs to carry out this manipulation at least 3-4 times a day.
In addition, the patient must assess his condition, control his diet and amount of physical activity, and also regularly visit the attending physician, who, in addition to talking with the patient, must examine his legs and measure blood pressure. Once a year, a patient with type 1 diabetes must undergo all the necessary tests (biochemical blood test, general blood and urine test, determination of the level of glycosylated hemoglobin), undergo an examination by an ophthalmologist and neurologist, and have a chest x-ray.
Prevention of development
The development of type 1 diabetes mellitus in people with a high genetic predisposition can be prevented by preventing intrauterine viral infections, as well as contracting viral infections in childhood and adolescence.
You should not include in the diet of children predisposed to the disease, nutritional formulas containing gluten, foods with preservatives and dyes that can cause an autoimmune reaction against insulin-producing cells of the pancreas.
Source: http://www.endoinfo.ru/theory_pacients/sakharnyy-diabet/sakharnyy-diabet-1-tipa.html
Type 1 diabetes mellitus in adults
Diabetes mellitus is a major medical and social problem throughout the world. This is explained by its wide distribution, the severity of late complications, and the high cost of diagnostic and treatment tools that patients need throughout their lives.
According to experts from the World Health Organization, the total number of patients with all forms of diabetes mellitus today is over 160 million people. Every year, the number of newly diagnosed cases is 6–10% of the total number of patients, thus the number of people suffering from this disease doubles every 10–15 years.
Type 1 diabetes is the most severe form of diabetes, accounting for no more than 10% of all cases of the disease. The highest incidence is observed in children aged 10 to 15 years - 40.0 cases per 100 thousand people.
An international expert committee, founded in 1995 with the support of the American Diabetes Association, proposed a new classification, which is accepted in most countries of the world as a recommendation document. The main idea underlying the modern classification of diabetes is a clear identification of the etiological factor in the development of diabetes.
Type 1 diabetes mellitus is a metabolic (metabolic) disease characterized by hyperglycemia, which is based on the destruction of β-cells, leading to an absolute deficiency of insulin. This form of diabetes was previously referred to as insulin-dependent diabetes mellitus or juvenile diabetes mellitus.
The destruction of β-cells in most cases among the European population is of an autoimmune nature (with the participation of the cellular and humoral components of the immune system) and is caused by the congenital absence or loss of tolerance to β-cell autoantigens.
Multiple genetic predisposing factors lead to autoimmune destruction of β-cells. The disease has a clear association with the HLA system, with the DQ A1 and DQ B1 genes, as well as DR B1. HLA DR/DQ alleles can be both predisposing and protective.
Type 1 diabetes is often combined with other autoimmune diseases, such as Graves' disease (diffuse toxic goiter), autoimmune thyroiditis, Addison's disease, vitiligo and pernicytic anemia. Type 1 diabetes may be a component of an autoimmune syndrome complex (autoimmune polyglandular syndrome type 1 or 2, “rigid person” syndrome).
Attention!
Summarizing the clinical and experimental data obtained to date, we can present the following concept of the pathogenesis of type 1 diabetes. Despite the appearance of an acute onset, type 1 diabetes develops gradually. The latent period can last for several years. Clinical symptoms appear only after 80% of β-cells have been destroyed.
An autopsy study of pancreatic tissue from patients with type 1 diabetes reveals the phenomena of insulitis - a specific inflammation characterized by infiltration of islets with lymphocytes and monocytes.
The earliest stages of the preclinical period of type 1 diabetes are characterized by the appearance of clones of autoreactive T lymphocytes that produce cytokines, which leads to the destruction of β-cells. Insulin, glutamate decarboxylase, heat-shock protein 60, and fogrin are currently considered as putative primary autoantigens that, under certain conditions, cause proliferation of cytotoxic T-lymphocytes.
In response to the destruction of β-cells, plasma cells secrete autoantibodies to various β-cell antigens, which are not directly involved in the autoimmune reaction, but indicate the presence of an autoimmune process. These autoantibodies belong to the immunoglobulin G class and are considered as immunological markers of autoimmune damage to β-cells.
There are islet cell autoantibodies (ICA - a set of autoantibodies to various cytoplasmic antigens of the β-cell), β-cell-specific autoantibodies to insulin, antibodies to glutamate decarboxylase (GAD), to phosphotyrosine phosphatase (IA-2), and fogrin.
Autoantibodies to β-cell antigens are the most important markers of autoimmune destruction of β-cells and they appear in typical type 1 diabetes much earlier than the clinical picture of diabetes develops. Autoantibodies to islet cells appear in the serum 5–12 years before the first clinical manifestations of diabetes mellitus, their titer increases in the late stage of the preclinical period.
There is also idiopathic type 1 diabetes mellitus, in which there is a decrease in β-cell function with the development of symptoms of insulinopenia, including ketosis and ketoacidosis, but there are no immunological markers of autoimmune destruction of β-cells. This subtype of diabetes mellitus occurs mainly among patients of African or Asian race. This form of diabetes mellitus has a clear inheritance. The absolute need for replacement therapy in such patients may appear and disappear over time.
As population studies have shown, type 1 diabetes among the adult population is much more common than previously thought. In 60% of cases, type 1 diabetes develops after age 20. The onset of diabetes in adults can have a different clinical picture. The literature describes the asymptomatic development of type 1 diabetes in first- and second-degree relatives of patients with type 1 diabetes with a positive titer of autoantibodies to β-cell antigens, when the diagnosis of diabetes mellitus was made only based on the results of an oral glucose tolerance test.
The classic course of type 1 diabetes with the development of a state of ketoacidosis at the onset of the disease also occurs in adults. The development of type 1 diabetes has been described in all age groups, up to the ninth decade of life.
In typical cases, the onset of type 1 diabetes has pronounced clinical symptoms, reflecting a deficiency of insulin in the body. The main clinical symptoms are: dry mouth, thirst, frequent urination, weight loss. Quite often the onset of the disease is so acute that patients can pinpoint the month, and sometimes even the day, when they first experienced the above symptoms.
Rapid, sometimes up to 10–15 kg per month, loss of body weight for no apparent reason is also one of the main symptoms of type 1 diabetes. In some cases, the onset of the disease is preceded by a severe viral infection (influenza, mumps, etc.) or stress. Patients complain of severe weakness and fatigue. Autoimmune diabetes mellitus usually begins in children and adolescents, but can develop at any age.
If symptoms of diabetes mellitus are present, laboratory tests are necessary to confirm the clinical diagnosis. The main biochemical signs of type 1 diabetes are: hyperglycemia (as a rule, a high percentage of sugar in the blood is determined), glucosuria, ketonuria (the presence of acetone in the urine). In severe cases, decompensation of carbohydrate metabolism leads to the development of diabetic ketoacidotic coma.
Diagnostic criteria for diabetes mellitus:
- fasting plasma glucose more than 7.0 mmol/l (126 mg%);
- fasting capillary blood glucose more than 6.1 mmol/l (110 mg%);
- plasma glucose (capillary blood) 2 hours after a meal (or a load of 75 g of glucose) more than 11.1 mmol/l (200 mg%).
Determining the level of C-peptide in serum allows one to assess the functional state of β-cells and, in doubtful cases, to distinguish type 1 diabetes from type 2 diabetes. Measuring C-peptide levels is more informative than insulin levels. In some patients at the onset of type 1 diabetes, a normal basal level of C-peptide may be observed, but there is no increase in it during stimulation tests, which confirms the insufficient secretory ability of β-cells.
The main markers confirming the autoimmune destruction of β-cells are autoantibodies to β-cell antigens: autoantibodies to GAD, ICA, insulin. Autoantibodies to islet cells are present in the serum of 80–95% of patients with newly diagnosed type 1 diabetes and in 60–87% of individuals in the preclinical period of the disease.
The progression of β-cell destruction in autoimmune diabetes mellitus (type 1 diabetes) may vary. In childhood, the loss of β-cells occurs rapidly and by the end of the first year of the disease the residual function fades away. In children and adolescents, the clinical manifestation of the disease usually occurs with symptoms of ketoacidosis. However, in adults there is also a slowly progressive form of type 1 diabetes mellitus, described in the literature as slowly progressive autoimmune diabetes of adults - Latent Autoimmune Diabetes in Adults (LADA).
Slowly progressive autoimmune diabetes of adults (LADA)
This is a special variant of the development of type 1 diabetes mellitus observed in adults. The clinical picture of type 2 diabetes and LADA at the onset of the disease is similar: compensation of carbohydrate metabolism is achieved through diet and/or the use of oral hypoglycemic drugs, but then, during a period that can last from 6 months to 6 years, decompensation of carbohydrate metabolism is observed and insulin requirement develops. A comprehensive examination of such patients reveals genetic and immunological markers characteristic of type 1 diabetes.
LADA is characterized by the following features:
- age of debut, usually exceeding 25 years;
- clinical picture of type 2 diabetes without obesity;
- initially, satisfactory metabolic control achieved through the use of diet and oral hypoglycemic drugs;
- development of insulin requirements in the period from 6 months to 10 years (on average from 6 months to 6 years);
- presence of markers of type 1 diabetes: low level of C-peptide; the presence of autoantibodies to β-cell antigens (ICA and/or GAD); presence of HLA alleles at high risk of developing type 1 diabetes.
As a rule, patients with LADA do not have a clear clinical picture of the onset of type I diabetes, which is typical for children and adolescents. At onset, LADA is “masked” and initially classified as type 2 diabetes because the process of autoimmune β-cell destruction in adults may be slower than in children.
Attention!
Symptoms of the disease are erased, there is no pronounced polydipsia, polyuria, weight loss and ketoacidosis. Excess body weight also does not exclude the possibility of developing LADA. The function of β-cells fades slowly, sometimes over several years, which prevents the development of ketoacidosis and explains the satisfactory compensation of carbohydrate metabolism when taking PSSP in the first years of the disease.
In such cases, type 2 diabetes is mistakenly diagnosed. The gradual nature of the development of the disease leads to the fact that patients seek medical help too late, having time to adapt to the developing decompensation of carbohydrate metabolism. In some cases, patients come to the doctor 1–1.5 years after the manifestation of the disease. In this case, all the signs of a sharp insulin deficiency are revealed: low body weight, high glycemia, lack of effect from PSSP.
P. Z. Zimmet (1999) gave the following definition of this subtype of type 1 diabetes: “Autoimmune diabetes that develops in adults may not be clinically different from type 2 diabetes, and is manifested by a slow deterioration of metabolic control with the subsequent development of insulin dependence.” At the same time, the presence in patients of the main immunological markers of type 1 diabetes - autoantibodies to β-cell antigens, along with low basal and stimulated levels of C-peptide, allows a diagnosis of slowly progressing autoimmune diabetes of adults.
Main diagnostic criteria for LADA:
- presence of autoantibodies to GAD and/or ICA;
- low basal and stimulated C-peptide levels;
- presence of HLA alleles at high risk for type 1 diabetes.
The presence of autoantibodies to β-cell antigens in patients with a clinical picture of type II diabetes at the onset of the disease has a high prognostic value regarding the development of insulin need. The results of the UK Prospective Diabetes Study (UKPDS), which examined 3672 patients with an initial diagnosis of type 2 diabetes, showed that antibodies to ICA and GAD have the greatest prognostic value in young patients ().
According to P. Zimmet, the prevalence of LADA is about 10–15% among all patients with diabetes mellitus and about 50% of cases occur in type 2 diabetes without obesity.
The results of our study showed that patients aged 30 to 64 years, who at the onset of the disease had a clinical picture of type 2 diabetes without obesity, a significant decrease in body weight (15.5 ± 9.1 kg) and concomitant autoimmune thyroid diseases (TDD) or AIT) represent a group at increased risk of developing LADA.
Determination of autoantibodies to GAD, ICA and insulin in this category of patients is necessary for timely diagnosis of LADA. Most often in LADA, antibodies to GAD are detected (according to our data, in 65.1% of patients with LADA), compared with antibodies to ICA (in 23.3% of LADA) and to insulin (in 4.6% of patients). The presence of a combination of antibodies is not typical. The titer of antibodies to GAD in patients with LADA is lower than in patients with type 1 diabetes with the same duration of the disease.
LADA patients represent a high-risk group for developing insulin requirements and require timely administration of insulin therapy. The OGTT results indicate the absence of stimulated insulin secretion in 46% of LADA patients and its decrease in 30.7% of patients already in the first 5 years of the disease.
The absence of obesity, decompensation of carbohydrate metabolism when taking PSSP, low basal levels of insulin and C-peptide in LADA patients indicate a high probability of absence of stimulated insulin secretion and the need for insulin administration.
If patients with LADA have a high degree of insulin resistance and insulin hypersecretion in the first years of the disease, it is recommended to prescribe drugs that do not deplete the function of β-cells, but improve the peripheral sensitivity of tissues to insulin, for example biguanides or glitazones (actos, avandia). Such patients are usually overweight and have satisfactory compensation of carbohydrate metabolism, but require further observation.
To assess peripheral insulin resistance, the insulin resistance index can be used - Homa-IR = ins0/22.5 eLnglu0 (where ins0 is the fasting insulin level and glu0 is fasting plasma glucose) and/or the index of general tissue sensitivity to insulin (ISI - insulin sensitivity index, or Matsuda index*), obtained based on the results of the OGTT.
With normal glucose tolerance, Homa-IR is 1.21–1.45 points; in patients with type 2 diabetes, the Homa-IR value increases to 6 and even 12 points. The Matsuda index in the group with normal glucose tolerance is 7.3±0.1 UL–1 x ml x mg–1 x ml, and in the presence of insulin resistance its values decrease.
Preserving one's own residual insulin secretion in patients with type 1 diabetes mellitus is very important, since it is noted that in these cases the disease is more stable, and chronic complications develop more slowly and later.
The issue of the importance of C-peptide in the development of late complications of diabetes mellitus is discussed. It was found that in the experiment, C-peptide improves kidney function and glucose utilization. It was found that infusion of small doses of biosynthetic C-peptide can affect microcirculation in human muscle tissue and renal function.
To determine LADA, more widespread immunological studies are indicated among patients with type 1 diabetes, especially in the absence of obesity and early ineffectiveness of PSSP. The main diagnostic method is the determination of autoantibodies to GAD and to ICA.
A special group of patients who also require close attention and where there is a need to determine autoantibodies to GAD and ICA are women with gestational diabetes mellitus (GDM). It has been established that 2% of women with gestational diabetes mellitus develop type 1 diabetes within 15 years.
The etiopathogenetic mechanisms of the development of GDM are very heterogeneous, and for the doctor there is always a dilemma: is GDM the initial manifestation of type 1 or type 2 diabetes. McEvoy et al. published data on the high incidence of autoantibodies to ICA among Native and African-American women in America. According to other data, the prevalence of autoantibodies to ICA and GAD was 2.9 and 5%, respectively, among Finnish women with a history of GDM.
Thus, patients with GDM may experience a slow development of insulin-dependent diabetes mellitus, as with LADA diabetes. Screening patients with GDM to determine autoantibodies to GAD and ICA makes it possible to identify patients who require insulin administration, which will make it possible to achieve optimal compensation of carbohydrate metabolism.
Taking into account the etiopathogenetic mechanisms of the development of LADA, the need for insulin therapy in these patients becomes obvious, while early insulin therapy is aimed not only at compensating carbohydrate metabolism, but also allows maintaining basal insulin secretion at a satisfactory level for a long period.
The use of sulfonylurea derivatives in LADA patients entails an increased load on β-cells and their faster depletion, while treatment should be aimed at maintaining residual insulin secretion and attenuating the autoimmune destruction of β-cells. In this regard, the use of secretogens in patients with LADA is pathogenetically unjustified.
Attention!
After clinical manifestation, most patients with a typical clinical picture of type 1 diabetes in a period of 1 to 6 months experience a transient decrease in insulin requirements associated with an improvement in the function of the remaining β-cells. This is the period of clinical remission of the disease, or “honeymoon”.
The need for exogenous insulin is significantly reduced (less than 0.4 units/kg body weight); in rare cases, even complete withdrawal of insulin is possible. The development of remission is a distinctive feature of the onset of type 1 diabetes and occurs in 18–62% of cases of newly diagnosed type 1 diabetes. The duration of remission ranges from several months to 3–4 years.
As the disease progresses, the need for exogenous increases and averages 0.7–0.8 IU/kg body weight. During puberty, the need for insulin can increase significantly - up to 1.0–2.0 U/kg body weight. With increasing duration of the disease due to chronic hyperglycemia, micro- (retinopathy, nephropathy, polyneuropathy) and macrovascular complications of diabetes mellitus (damage to coronary, cerebral and peripheral vessels) develop. The main cause of death is renal failure and complications of atherosclerosis.
Treatment
The goal of therapy for type 1 diabetes is to achieve target values of glycemia, blood pressure and blood lipid levels (), which can significantly reduce the risk of developing micro- and marcovascular complications and improve the quality of life of patients.
The results of the multicenter randomized Diabetes Control and Complication Trail (DCCT) trial have convincingly shown that good glycemic control reduces the incidence of diabetes complications. Thus, a decrease in glycohemoglobin (HbA1c) from 9 to 7% led to a reduction in the risk of developing diabetic retinopathy by 76%, neuropathy by 60%, and microalbuminuria by 54%.
Treatment of type 1 diabetes includes three main components:
- diet therapy;
- physical exercise;
- insulin therapy;
- training and self-control.
Diet therapy and exercise
When treating type 1 diabetes, foods containing easily digestible carbohydrates (sugar, honey, sweet confectionery, sweet drinks, jam) should be excluded from the daily diet. It is necessary to control the consumption (count bread units) of the following products: grains, potatoes, corn, liquid dairy products, fruits. The daily caloric intake should be covered by 55–60% from carbohydrates, 15–20% from proteins and 20–25% from fats, while the proportion of saturated fatty acids should be no more than 10%.
The physical activity regime should be purely individual. It should be remembered that physical exercise increases tissue sensitivity to insulin, reduces glycemic levels and can lead to the development of hypoglycemia. The risk of hypoglycemia increases during exercise and for 12–40 hours after prolonged strenuous exercise.
Light to moderate exercise lasting no more than 1 hour requires additional intake of easily digestible carbohydrates before and after exercise. With moderate long-term (more than 1 hour) and intense physical activity, adjustment of insulin doses is necessary. It is necessary to measure blood glucose levels before, during and after exercise.
Lifelong insulin replacement therapy is essential for the survival of patients with type 1 diabetes and plays a crucial role in the routine management of this disease. When prescribing insulin, different regimens can be used. Currently, it is customary to distinguish between traditional and intensified insulin therapy regimens.
The main feature of the traditional insulin therapy regimen is the lack of flexible adjustment of the dose of administered insulin to the glycemic level. In this case, self-monitoring of blood glucose is usually absent.
The results of multicenter DCCT convincingly proved the advantage of intensified insulin therapy in compensating carbohydrate metabolism in type 1 diabetes. Intensive insulin therapy includes the following:
- basal-bolus principle of insulin therapy (multiple injections);
- planned number of bread units for each meal (diet liberalization);
- self-monitoring (monitoring blood glucose throughout the day).
For the treatment of type 1 diabetes and the prevention of vascular complications, genetically engineered human insulins are the drugs of choice. Porcine and human semi-synthetic insulins obtained from pork are of lower quality compared to human genetically engineered ones.
Carrying out insulin therapy at this stage involves the use of insulins with different durations of action. To create a basic insulin level, intermediate-acting or long-acting insulins are used (approximately 1 unit per hour, which is an average of 24-26 units per day). In order to regulate the level of glycemia after meals, short-acting or ultra-short-acting insulins are used in a dose of 1-2 units per 1 bread unit.
Ultra-short-acting insulins (humalog, novorapid), as well as long-acting insulins (lantus) are insulin analogues. Insulin analogs are specially synthesized polypeptides that have the biological activity of insulin and have a number of specified properties.
These are the most promising insulin preparations in terms of intensified insulin therapy. Insulin analogues Humalog (lispro, Lilly), as well as novorapid (aspart, Novo Nordisk) are highly effective in regulating postprandial glycemia.
Their use also reduces the risk of hypoglycemia between meals. Lantus (insulin glargine, Aventis) is produced using recombinant DNA technology using a non-pathogenic laboratory strain of Escherichia coli (K12) as a producing organism and differs from human insulin in that the amino acid asparagine from position A21 is replaced by glycine and 2 molecules of arginine are added at C -end of the B-chain. These changes made it possible to obtain a peak-free, constant concentration profile of insulin action over 24 hours/day.
Ready-made mixtures of human insulins of various actions have been created, such as Mixtard (30/70), Insuman Comb (25/75, 30/70), etc., which are stable mixtures of short- and long-acting insulin in specified proportions.
To administer insulin, disposable insulin syringes are used (U-100 for administering insulin with a concentration of 100 U/ml and U-40 for insulin with a concentration of 40 U/ml), syringe pens (Novopen, Humapen, Optipen, Bd-pen, Plivapen) and insulin pumps. All children and adolescents with type 1 diabetes, as well as pregnant women with diabetes, patients with impaired vision and lower limb amputations due to diabetes should be provided with syringe pens.
Achieving target glycemic values is impossible without regular self-monitoring and adjustment of insulin doses. Patients with type 1 diabetes need to independently monitor glycemia daily, several times a day, for which not only glucometers can be used, but also test strips for visual determination of blood sugar (Glucochrome D, Betachek, Suprima Plus).
To reduce the incidence of micro- and macrovascular complications of diabetes, it is important to achieve and maintain normal levels of lipid metabolism and blood pressure.
Source: https://www.lvrach.ru/2005/05/4532521/
Type 1 diabetes and heredity
Greetings! If you remember the day when you or your child was diagnosed with diabetes, you will also remember the questions that began to worry your fevered brain. I dare to assume that you never received an answer to the question: “Where did type 1 diabetes mellitus come from, if there was no one in your family with this disease?”, just like to the question: “Is type 1 diabetes mellitus inherited and /or what will happen to the rest of the children and family members?” They probably still bother you to this day.
Today I will try to answer these questions. Type 1 diabetes is a multifactorial and polygenic disease. You can never say which factor is leading or main. Some scientists divide type 1 diabetes into subtypes: A and B. By the way, type 1 diabetes is not the only form that can occur in the younger generation.
Subtype A is associated with autoimmune damage to the pancreas and the detection of antibodies confirms this. This subtype is most often detected in children and adolescents. But it happens that antibodies are not detected, but diabetes is present. In this case, we are talking about subtype B, which occurs for completely different reasons not related to the functioning of the immune system. To date, these causes are not known, and therefore diabetes is called idiopathic.
Genetic research
One thing is clear that type 1 is a disease with a hereditary predisposition. What does this mean and how does it differ from just a hereditary disease? The fact is that a hereditary disease is the transmission of a gene from generation to generation or a mutation of a gene in a future organism. In this case, a new person is already born with a pathology or some other defect.
In the case of diabetes, everything is more complicated. There are certain genes and gene sections (I will speak in simplified terms), which, when combined in a certain way during the meeting of the egg and the sperm, increase the risk of developing type 1 diabetes. In other words, it is not the defective gene that is inherited, but the degree of risk for the disease.
And for the disease to materialize, that is, to develop, provoking factors and a high degree of risk are necessary. If you conduct a genetic study, you can identify a certain degree of risk, which can be high, medium and low. Therefore, it is not at all necessary that if a person is at risk of developing type 1 diabetes, he will get it. Most often, the development of diabetes is associated with the following genes or gene regions - HLA DR3, DR4 and DQ.
In this regard, it does not matter at all if you have no known history of type 1 diabetes in your family now or in past generations. It is entirely possible that your ancestors had a low risk that never materialized. And besides this, how well do you know your family tree? What did children and adults die from at a young age? After all, diagnostics 100 years ago were not the most progressive, and doctors were not often consulted, especially in rural areas.
Therefore, I believe that it is completely pointless to look for those responsible for the spread of diabetes. Moreover, you should not reproach yourself (I am addressing the parents) for missing, not watching and not saving the child. To ease your guilt, I will say that the autoimmune process occurs long before the clinical manifestations of diabetes, about several years, and in some cases, ten years.
Since then, a lot of water has flowed under the bridge and it is difficult to remember who is to blame for what. In the end, no matter how much we want, we cannot protect ourselves or our children from everything bad. Bad things happen and if this happened, then let's think that this is FATE, which cannot be deceived.
Immune research
When a family has a relative with type 1 diabetes, then to predict the incidence of diabetes in other family members, not only a genetic study is used, but also the determination of autoantibodies, i.e. antibodies that fight against the tissues of one’s own body. For example, if an older child has type 1 diabetes, parents may want to do genetic and antibody testing on the younger child to identify diabetes risks because antibodies appear long before the child shows signs of diabetes.
- antibodies to islet beta cells - ICA (detected in 60-80% of cases) When combined with GAD, it sharply increases the risk of developing diabetes, but in isolation the risk of diabetes is small.
- anti-insulin antibodies - IAA (detected in 30-60% of cases) In isolated form, has little effect on the development of diabetes; the risk increases in the presence of any other antibodies.
- antibodies to glutamate decarboxylase - GAD (detected in 80-95% of cases) Increases the risk of developing diabetes, even in isolated form.
But here, too, everything is ambiguous. The detection of any one group of antibodies in a child does not mean at all that he will develop diabetes in the future. This only means that this child has a high risk of developing diabetes, which may not materialize. And then, no one is safe from a laboratory error, so it is recommended to retake the tests after 1-2 months.
Therefore, I do not recommend testing for antibodies in healthy family members. IMHO. What can you do if you know you have antibodies? Of course, you can get into experimental groups where they test methods for preventing diabetes in high-risk groups, but would you want to subject a still healthy child to unknown manipulations? Personally, I’m not ready, and we live far from the center of the country.
Apart from unnecessary hassle, these actions do not bring anything good. Constant expectations and thoughts may one day come true. Personally, I believe that our thoughts are material and everything we think about will someday come true. Therefore, you don’t need to think about the bad, attract only positive thoughts that everything will be fine and all other family members will be healthy.
The only thing that can be done is to periodically determine fasting glucose and/or glycated hemoglobin so as not to miss the manifestation of diabetes. Because so far there are no proven methods that 100% prevent the development of diabetes, and there are none at all.
Another question that worries everyone with type 1 diabetes: “What are the risks of illness in children whose parents have diabetes or if there is already a child with diabetes in the family?” A 16-year study was recently completed that examined the prognosis of the disease in the families of patients. Here are his results.
The risk of developing diabetes without a known relative with diabetes is only 0.2 - 0.4%. The greater the number of relatives with diabetes in a family, the higher the risk. The risk of developing diabetes for family members of a person with type 1 diabetes is on average 5%. If two children in a family are sick, the risk for the third is 9.5%.
If two parents are sick, then the risk of developing type 1 diabetes for the child already increases to 34%. In addition, the risk of developing type 1 diabetes depends on the age at which the disease manifests itself in the patient. The earlier a child in the family gets sick, the higher the risk for the second one. If the manifestation of the disease occurred before the age of 20, then the risk for the second child is 6.4%, and if the manifestation of the disease is older than 20 years, then the risk is 1.2%.
Prevention
But what can be done to reduce the influence of these notorious factors that trigger the autoimmune process? And although it all comes down to “lucky or unlucky,” you can still try to influence them as much as possible. Here is a list of methods for the primary prevention of type 1 diabetes.
- Prevention of intrauterine infection and viral infections of the mother during pregnancy.
- Prevention of certain viral infections in children and adolescents, such as rubella, measles, mumps, enteroviruses, chicken pox, influenza.
- Timely treatment of chronic foci of infection (sinusitis, carious teeth, etc.).
- Carrying out timely vaccination, strictly according to the rules and proven vaccines.
- Excluding cow's milk protein from the diet of infants.
- Long-term breastfeeding (minimum 18 months).
- Excluding the introduction of complementary foods containing gluten-containing products under the age of one year.
- Exclusion from the diet of foods containing nitrates, preservatives and dyes.
- Normal intake of vitamin D.
- Adding Omega 3 fatty acid supplements to your diet.
- Reducing the consumption of fast carbohydrates due to excessive stress on the pancreas.
In conclusion, I want to say. We are all different, with varying degrees of anxiety and “not giving a fuck.” Therefore, it’s up to you to decide whether to have your child diagnosed with diabetes or go yourself. Ask yourself: “Are you ready for a positive outcome? Are you ready to find out that your child is at risk of developing this disease and at the same time continue to live peacefully? If yes, then you can undergo a complete genetic and immune examination.
If you have type 1 diabetes, your pancreas cannot create insulin. This vital hormone helps your body's cells convert sugar into energy.
Without it, sugar builds up in the blood and can reach dangerous levels. To avoid life-threatening complications, people with type 1 diabetes must take insulin throughout their lives.

Symptoms of type 1 diabetes usually begin suddenly and may include:
- Greater than usual thirst;
- Dry mouth;
- Fruity smell in exhaled air;
- Increased diuresis.
Later symptoms

If blood sugar levels remain high, type 1 diabetes often results in:
- Weight loss;
- Increased appetite;
- Lack of energy, drowsiness.

Many people with type 1 diabetes develop troublesome skin conditions, including:
- Bacterial infections;
- Fungal infections;
- Itchy, dry skin, poor circulation.
Girls with type 1 diabetes are more likely to experience genital fungal infections. Babies can develop candidiasis, a severe form of diaper rash caused by fungi. It can easily spread from the diaper area to the thighs and abdomen.
Dangerous complications

If your blood glucose levels are not controlled, type 1 diabetes can lead to more severe symptoms, such as:
- Numbness or tingling in the legs;
- Deterioration of vision;
- Low blood glucose levels (called hypoglycemia);
- Loss of consciousness.
If your blood sugar levels are too high or too low, you may develop a diabetic coma. You may not have any warning signs before this happens. In this case, emergency medical attention is required.

Without treatment, type 1 diabetes deprives your cells of the sugar they need for energy. Instead, your body begins to burn fat, which leads to the formation and accumulation of ketones in the blood. These are acids that can poison your body.
This—and other changes in your blood—can trigger a life-threatening condition called ketoacidosis. This situation requires quick and urgent medical attention. You may need to be admitted to hospital.
Comparison of diabetes mellitus type 1 and 2

- In type 1 diabetes, your immune system destroys the cells in your pancreas that produce insulin.
- In type 2 diabetes, the pancreas is not affected. It usually produces enough insulin, but your body does not use it properly.
The symptoms of both forms are the same, but tend to develop more quickly in people with type 1 diabetes.
What are the causes of type 1 diabetes

Doctors don't know exactly what exactly causes your immune system to attack your pancreas. Scientists have discovered 50 genes or gene sequences that make you more likely to develop type 1 diabetes.
But this in itself does not mean that this will happen. Some researchers believe that environmental triggers also play an important role. These factors may include a virus or changes that occur in your body during pregnancy.
Who develops type 1 diabetes?

Type 1 diabetes can appear at any time in life. But in most cases, this disease is diagnosed before the age of 19.
It affects both boys and girls equally, and is more common in whites than other ethnic groups. According to the World Health Organization, type 1 diabetes is rare in African, Indian and Asian peoples.

Your doctor will check your fasting blood sugar or may do a random blood sugar test. He may also order a glycosylated hemoglobin test, which will show your average blood glucose values over the past 2-3 months.
Tests should be repeated on two separate days. A more complex glucose tolerance test will also help your doctor make a diagnosis.
Long-term problems

Having high blood glucose levels for a long time can damage many of your body's systems. Type 1 diabetes can also make you more likely to develop the following diseases:
- Heart disease and stroke;
- Kidney failure;
- Blindness and other vision problems;
- Gum disease and gum loss;
- Damage to the nerves of the arms, legs and organs.
Check your blood sugar levels

The first step to preventing complications from developing is to monitor your blood glucose levels, such as with a glucose meter. To do this, you need to prick your finger, apply a drop of blood to a test strip, and insert it into the meter. The results will help you stick to your treatment plan. When glucose levels are close to normal, you will have more energy, fewer skin problems, and a lower risk of heart disease and kidney damage.
Can continuous glucose monitoring help?
This device uses a sensor to measure your body's glucose levels every 10 seconds. It sends information to a mobile phone-sized monitor that you carry.

The system automatically records average values for up to 72 hours. The device is not intended for daily testing of sugar levels or long-term independent use. It does not replace a regular blood glucose test, but is used to determine patterns in blood glucose levels.
Treatment with insulin injections

Every patient with type 1 diabetes should take insulin. Most people take it by injection and need several shots each day. Your doctor will tell you how to adjust your dose based on your blood glucose meter results. The goal is to keep these levels within normal values as often as possible.

Hypoglycemia occurs when insulin reduces blood glucose levels to dangerous levels. It can be mild, moderate or severe. Danger signs include:
- Severe drowsiness or yawning;
- Inability to speak or think clearly;
- Loss of muscle coordination;
- Sweating, tremors, pallor;
- Convulsions;
- Loss of consciousness.

Carry at least 15 grams of easily digestible carbohydrates with you at all times. They will quickly raise blood glucose levels to combat hypoglycemia.
Some examples of them:
- Half a cup of fruit juice or non-diet soda;
- 1 cup milk;
- 2 tablespoons raisins;
- 3 glucose tablets or 5 caramels.
If your blood sugar is still too low after 15 minutes, eat another 15 grams of these carbohydrates.
If you lose consciousness, you may need help from people around you. Wear a medical bracelet that indicates you have diabetes and a glucagon syringe kit. This medication may be injected under your skin. Tell your friends and family how to recognize the signs of hypoglycemia and show them how to give you the shot.

This device may reduce the chances of developing hypoglycemia. It releases insulin around the clock through a tiny tube inserted into your skin. You no longer need injections. An insulin pump can help keep your blood sugar levels stable and can give you more freedom in your dietary choices. There are some disadvantages, so ask your doctor if this option is right for you.
Most likely, your doctor will advise you to get tested for glycosylated hemoglobin every 3-6 months.

This will show how well your blood sugar levels have been controlled over the past 2-3 months. If the test results are bad, you may need to adjust your insulin dose or modify your diet or physical activity.
If insulin injections fail to control your blood glucose or you frequently experience hypoglycemia, your doctor may suggest a pancreatic islet cell transplant.

This is an experimental surgery in which the surgeon transplants healthy insulin-producing cells from a donor into your pancreas. But there is a downside - the results may only last a few years. And you will need to take anti-rejection medications, which can have serious side effects.

Researchers continue to work on a system called an artificial pancreas. It is a combination of an insulin pump and continuous glucose monitoring, which is controlled by a complex computer program. The goal is for it to work like a real pancreas. This means that it will regulate the amount of insulin released in response to rising or falling blood glucose levels. Early research suggests that an artificial pancreas may improve blood sugar control.
Exercise with caution
You should be physically active, but be careful while exercising. To avoid a sudden drop in blood glucose levels, your doctor may advise
Before exercising, you should do the following:
- Check your glucose levels;
- Adjust your insulin dose;
- Have a snack.
Your doctor may also recommend testing your urine for ketones, a sign that your blood glucose levels are very high. Avoid strenuous exercise when you have these substances in your urine.

There are many myths about what people with diabetes can and cannot eat. The reality is that no foods are completely prohibited. You can consume sweets as part of a well-balanced diet and treatment plan. It is important to work with your doctor to balance your insulin injections, diet, and physical activity.

Tell your doctor that you are planning to become pregnant. If your diabetes is not well controlled, it can cause complications, including birth defects in the baby. Good blood glucose control before conception reduces your chances of developing these problems and reduces the risk of miscarriage. In addition, the risk of eye damage and dangerous spikes in blood pressure will also be reduced.

When a child is diagnosed with diabetes, it affects the entire family. Parents should help him in determining blood glucose levels, planning meals and adjusting insulin dosage. This condition requires 24-hour care, so you should also schedule treatment while attending school. Find out who at your child's school can give you an insulin injection if needed.
Dear visitors of the Farmamir website. This article does not constitute medical advice and should not serve as a substitute for consultation with a physician.